Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones
- PMID: 9625875
- PMCID: PMC2231010
- DOI: 10.1111/j.1469-7793.1998.165bz.x
Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones
Abstract
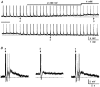
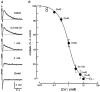
1. The effects of Cs+ on the action potential, post-train after-hyperpolarization (AHP), Ca2+-dependent post-spike depolarizing after-potential (DAP) and phasic firing were examined during intracellular recordings from magnocellular neurosecretory cells (MNCs) in superfused rat hypothalamic explants. 2. Extracellular Cs+ reversibly inhibited (IC50, approximately 1 mM) DAPs, and associated after-discharges, that followed brief spike trains in each of sixteen cells tested. Although bath application of Cs+ also provoked a small reversible depolarization, inhibition of the DAP was retained when membrane voltage was kept constant by current injection. 3. Application of Cs+ had no significant effects on spike duration (n = 8), frequency-dependent spike broadening (n = 8), spike hyperpolarizing after-potentials (n = 14), or the amplitude of the isolated AHP (n = 7). Caesium-evoked inhibition of the DAP, therefore, does not result from diminished spike-evoked Ca2+ influx, and may reflect direct blockade of the conductance underlying the DAP. 4. Inhibition of the DAP was associated with an enhancement of the amplitude and duration of the AHP, indicating that the currents underlying the AHP and the DAP overlap in time following a train of action potentials, and that the relative magnitude of these currents is an important factor in determining the shape and time course of post-train after-potentials. 5. Bath application of Cs+ reversibly abolished phasic firing in each of seven cells tested. This effect was reversible and persisted at all subthreshold voltages tested. These results indicate that the current underlying the DAP is necessary for the genesis of plateau potentials and phasic firing in MNCs.
Figures


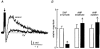


Similar articles
-
Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones.J Physiol. 2002 Dec 1;545(2):537-42. doi: 10.1113/jphysiol.2002.033589. J Physiol. 2002. PMID: 12456832 Free PMC article.
-
Intracellular study of calcium-related events in cat magnocellular neuroendocrine cells.J Physiol. 1991 Mar;434:337-49. doi: 10.1113/jphysiol.1991.sp018473. J Physiol. 1991. PMID: 2023122 Free PMC article.
-
Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro.J Physiol. 1996 Jul 15;494 ( Pt 2)(Pt 2):389-98. doi: 10.1113/jphysiol.1996.sp021500. J Physiol. 1996. PMID: 8841999 Free PMC article.
-
Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro.J Physiol. 1998 Dec 1;513 ( Pt 2)(Pt 2):493-506. doi: 10.1111/j.1469-7793.1998.493bb.x. J Physiol. 1998. PMID: 9806998 Free PMC article.
-
Extrinsic modulation of spike afterpotentials in rat hypothalamoneurohypophysial neurons.Cell Mol Neurobiol. 1998 Feb;18(1):3-12. doi: 10.1023/a:1022566924921. Cell Mol Neurobiol. 1998. PMID: 9524726 Free PMC article. Review.
Cited by
-
Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility.J Neurosci. 2006 Nov 15;26(46):11961-73. doi: 10.1523/JNEUROSCI.3171-06.2006. J Neurosci. 2006. PMID: 17108170 Free PMC article.
-
Measuring spike coding in the rat supraoptic nucleus.J Physiol. 2004 Feb 15;555(Pt 1):281-96. doi: 10.1113/jphysiol.2003.053264. Epub 2003 Nov 7. J Physiol. 2004. PMID: 14608010 Free PMC article.
-
A reduction in SK channels contributes to increased activity of hypothalamic magnocellular neurons during heart failure.J Physiol. 2017 Oct 15;595(20):6429-6442. doi: 10.1113/JP274730. Epub 2017 Aug 2. J Physiol. 2017. PMID: 28714070 Free PMC article.
-
Electrophysiological properties of identified oxytocin and vasopressin neurones.J Neuroendocrinol. 2019 Mar;31(3):e12666. doi: 10.1111/jne.12666. Epub 2019 Feb 14. J Neuroendocrinol. 2019. PMID: 30521104 Free PMC article. Review.
-
Activity-dependent synaptic plasticity in the supraoptic nucleus of the rat hypothalamus.J Physiol. 2006 Jun 15;573(Pt 3):711-21. doi: 10.1113/jphysiol.2006.109447. Epub 2006 Apr 13. J Physiol. 2006. PMID: 16613872 Free PMC article.
References
-
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. - PubMed
-
- Andrew RD, Dudek FE. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. Journal of Neurophysiology. 1984a;51:552–566. - PubMed
-
- Andrew RD, Dudek FE. Spike broadening in magnocellular neuroendocrine cells of rat hypothalamic slices. Brain Research. 1985;334:176–179. 10.1016/0006-8993(85)90583-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

