Neuroeffector transmission in arterioles of the guinea-pig choroid
- PMID: 9625878
- PMCID: PMC2231030
- DOI: 10.1111/j.1469-7793.1998.209bz.x
Neuroeffector transmission in arterioles of the guinea-pig choroid
Abstract
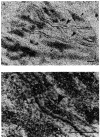
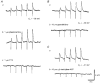
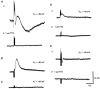
1. Using conventional microelectrode techniques, membrane potentials were recorded from smooth muscle cells of guinea-pig choroidal arterioles. 2. Transmural stimulation initiated excitatory junction potentials (EJPs) which were abolished by either guanethidine or alpha,beta-methylene-ATP but not by phentolamine, indicating that they resulted from activation of purinoceptors. 3. Trains of stimuli evoked EJPs which were followed by a slow depolarization, an inhibitory junction potential (IJP) or a biphasic membrane potential change which consisted of an IJP and a subsequent slow depolarization. 4. Slow depolarizations were abolished by either phentolamine or guanethidine, indicating that they resulted from activation of alpha-adrenoceptors. 5. IJPs were abolished by atropine but not by guanethidine, and were reduced by 50 % by apamin with the residual response being abolished by charybdotoxin, indicating that they resulted from the activation of muscarinic receptors which open two sets of Ca2+-activated K+ channels. 6. Most responses were followed by slow hyperpolarizations. These were almost abolished by L-nitroarginine, an effect which was partly overcome by L-arginine, and were abolished by glibenclamide, indicating that they resulted from the release of NO and activation of ATP-sensitive K+ channels. 7. Immunohistochemical analysis showed that arterioles were densely innervated by adrenergic nerve fibres. A population of fibres, likely to be cholinergic, was also identified. Furthermore, populations of nerve fibres immunoreactive to antibodies against either nitric oxide synthase (NOS) or substance P (SP) were also identified. 8. These findings indicate that choroidal arterioles of the guinea-pig are innervated by at least three different populations of nerves, adrenergic nerves which evoke excitatory responses, cholinergic nerves which evoke inhibitory responses and a population of nerves which cause the release of NO.
Figures

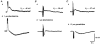





References
-
- Albert A. Ocular circulation. In: Hart WM Jr, editor. Adler's Physiology of the Eye. 9. St Louis, MO, USA: Mosby-Year Book, Inc.; 1992. pp. 198–227.
-
- Bolton TB, Large WA. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Quarterly Journal of Experimental Physiology. 1986;89:163–171. - PubMed
-
- Clarke A, Edwards FR, Hirst GDS, Silverberg GD. Developmental changes in the resting membrane potential of rat cerebral arteries. In: Mulvany MJ, Aalkjaer C, Heagerty AM, Nyborg NCB, Strandgaard S, editors. Resistance Arteries, Structure and Function. Amsterdam: Excerpta Medica; 1991. pp. 147–151.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

