Interaction of vault particles with estrogen receptor in the MCF-7 breast cancer cell
- PMID: 9628887
- PMCID: PMC2132791
- DOI: 10.1083/jcb.141.6.1301
Interaction of vault particles with estrogen receptor in the MCF-7 breast cancer cell
Abstract
A 104-kD protein was coimmunoprecipitated with the estrogen receptor from the flowtrough of a phosphocellulose chromatography of MCF-7 cell nuclear extract. mAbs to this protein identified several cDNA clones coding for the human 104-kD major vault protein. Vaults are large ribonucleoprotein particles of unknown function present in all eukaryotic cells. They have a complex morphology, including several small molecules of RNA, but a single protein species, the major vault protein, accounts for >70% of their mass. Their shape is reminiscent of the nucleopore central plug, but no proteins of known function have been described to interact with them. Western blot analysis of vaults purified on sucrose gradient showed the presence of estrogen receptor co-migrating with the vault peak. The AER317 antibody to estrogen receptor coimmunoprecipitated the major vault protein and the vault RNA also in the 20,000 g supernatant fraction. Reconstitution experiments of estrogen receptor fragments with the major vault protein mapped the site of the interaction between amino acids 241 and 280 of human estrogen receptor, where the nuclear localization signal sequences are located. Estradiol treatment of cells increased the amount of major vault protein present in the nuclear extract and coimmunoprecipitated with estrogen receptor, whereas the anti-estrogen ICI182,780 had no effect. The hormone-dependent interaction of vaults with estrogen receptor was reproducible in vitro and was prevented by sodium molybdate. Antibodies to progesterone and glucocorticoid receptors were able to coimmunoprecipitate the major vault protein. The association of nuclear receptors with vaults could be related to their intracellular traffic.
Figures
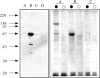


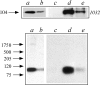




References
-
- Abbondanza C, De Falco A, Nigro V, Medici N, Armetta I, Molinari AM, Moncharmont B, Puca GA. Characterization and epitope mapping of a new panel of monoclonal antibodies to estradiol receptor. Steroids. 1993;58:4–12. - PubMed
-
- Ausubel, F.M., R. Breint, R.E. Kingston, D.D. Moore, J.G. Seidman, J.G. Smith, and K. Struhl. 1996. Current Protocols in Molecolar Biology. Vol. 3. Wiley & Sons Inc., New York.
-
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. - PubMed
-
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search for a plot. Cell. 1995;83:851–857. - PubMed
-
- Binart N, Chambraud B, Dumas B, Rowlands DA, Bigogne C, Levin JM, Garnier J, Baulieu EE, Catelli MG. The cDNA-derived amino acid sequence of chick heat shock protein Mr90,000 (HSP 90) reveals a “DNA like” structure: potential site of interaction with steroid receptors. Biochem Biophys Res Commun. 1989;159:140–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

