GalNAc-alpha-O-benzyl inhibits NeuAcalpha2-3 glycosylation and blocks the intracellular transport of apical glycoproteins and mucus in differentiated HT-29 cells
- PMID: 9628888
- PMCID: PMC2132799
- DOI: 10.1083/jcb.141.6.1311
GalNAc-alpha-O-benzyl inhibits NeuAcalpha2-3 glycosylation and blocks the intracellular transport of apical glycoproteins and mucus in differentiated HT-29 cells
Abstract
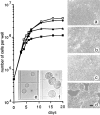
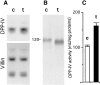
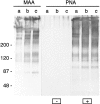
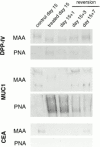
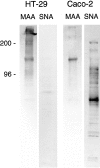
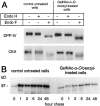
Exposure for 24 h of mucus-secreting HT-29 cells to the sugar analogue GalNAc-alpha-O-benzyl results in inhibition of Galbeta1-3GalNAc:alpha2,3-sialyltransferase, reduced mucin sialylation, and inhibition of their secretion (Huet, G., I. Kim, C. de Bolos, J.M. Loguidice, O. Moreau, B. Hémon, C. Richet, P. Delannoy, F.X. Real., and P. Degand. 1995. J. Cell Sci. 108:1275-1285). To determine the effects of prolonged inhibition of sialylation, differentiated HT-29 populations were grown under permanent exposure to GalNAc-alpha-O-benzyl. This results in not only inhibition of mucus secretion, but also in a dramatic swelling of the cells and the accumulation in intracytoplasmic vesicles of brush border-associated glycoproteins like dipeptidylpeptidase-IV, the mucin-like glycoprotein MUC1, and carcinoembryonic antigen which are no longer expressed at the apical membrane. The block occurs beyond the cis-Golgi as substantiated by endoglycosidase treatment and biosynthesis analysis. In contrast, the polarized expression of the basolateral glycoprotein GP 120 is not modified. Underlying these effects we found that (a) like in mucins, NeuAcalpha2-3Gal-R is expressed in the terminal position of the oligosaccharide species associated with the apical, but not the basolateral glycoproteins of the cells, and (b) treatment with GalNAc-alpha-O-benzyl results in an impairment of their sialylation. These effects are reversible upon removal of the drug. It is suggested that alpha2-3 sialylation is involved in apical targeting of brush border membrane glycoproteins and mucus secretion in HT-29 cells.
Figures






References
-
- Braulke T, Mach L, Hoflack B, Glossl J. Biosynthesis and endocytosis of lysosomal enzymes in human colon carcinoma SW1116 cells: impaired internalization of plasma membrane-associated cation-independent mannose 6-phosphate receptor. Arch Biochem Biophys. 1992;298:176–181. - PubMed
-
- Byrd, J.C., R. Dahiya, J. Huang, and Y.S. Kim. 1995. Inhibition of mucin synthesis by benzyl-α-GalNAc in KATO III gastric cancer and Caco-2 colon cancer cells. Eur. J. Cancer. 31A:1498–1505. - PubMed
-
- Capon C, Laboisse CL, Wieruszeki JM, Maoret JJ, Augeron C, Fournet B. Oligosaccharide structures of mucins secreted by the human colonic cancer cell line CL.16E. J Biol Chem. 1992;267:19248–19257. - PubMed
-
- Chang ML, Eddy RL, Shows TB, Lau JTY. Three genes that encode human β-galactosidase α-2,3-sialyltransferases. Structural analysis and chromosomal lapping studies. Glycobiology. 1995;5:319–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

