A calcium signaling cascade essential for myosin thick filament assembly in Xenopus myocytes
- PMID: 9628891
- PMCID: PMC2132793
- DOI: 10.1083/jcb.141.6.1349
A calcium signaling cascade essential for myosin thick filament assembly in Xenopus myocytes
Abstract
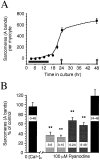
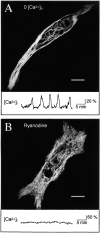
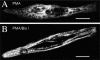
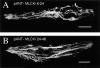
Spontaneous calcium release from intracellular stores occurs during myofibrillogenesis, the process of sarcomeric protein assembly in striated muscle. Preventing these Ca2+ transients disrupts sarcomere formation, but the signal transduction cascade has not been identified. Here we report that specific blockade of Ca2+ release from the ryanodine receptor (RyR) activated Ca2+ store blocks transients and disrupts myosin thick filament (A band) assembly. Inhibition of an embryonic Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) by blocking the ATP-binding site, by allosteric phosphorylation, or by intracellular delivery of a pseudosubstrate peptide, also disrupts sarcomeric organization. The results indicate that both RyRs and MLCK, which have well-described calcium signaling roles in mature muscle contraction, have essential developmental roles during construction of the contractile apparatus.
Figures








Similar articles
-
Calcium transients regulate patterned actin assembly during myofibrillogenesis.Dev Dyn. 2004 Feb;229(2):231-42. doi: 10.1002/dvdy.10428. Dev Dyn. 2004. PMID: 14745949
-
Calcium transients regulate titin organization during myofibrillogenesis.Cell Motil Cytoskeleton. 2005 Mar;60(3):129-39. doi: 10.1002/cm.20054. Cell Motil Cytoskeleton. 2005. PMID: 15662726
-
Effects of phosphorylation by myosin light chain kinase on the structure of Limulus thick filaments.J Cell Biol. 1991 May;113(3):563-72. doi: 10.1083/jcb.113.3.563. J Cell Biol. 1991. PMID: 2016336 Free PMC article.
-
Regulation of contractile activity in vascular smooth muscle by protein kinases.Rev Clin Basic Pharm. 1985 Jul-Dec;5(3-4):341-95. Rev Clin Basic Pharm. 1985. PMID: 3029813 Review.
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
Cited by
-
How to build a myofibril.J Muscle Res Cell Motil. 2005;26(6-8):343-54. doi: 10.1007/s10974-005-9016-7. J Muscle Res Cell Motil. 2005. PMID: 16465476
-
Spontaneous calcium transients manifest in the regenerating muscle and are necessary for skeletal muscle replenishment.Cell Calcium. 2014 Jul;56(1):34-41. doi: 10.1016/j.ceca.2014.04.004. Epub 2014 Apr 29. Cell Calcium. 2014. PMID: 24854233 Free PMC article.
-
Force Generation via β-Cardiac Myosin, Titin, and α-Actinin Drives Cardiac Sarcomere Assembly from Cell-Matrix Adhesions.Dev Cell. 2018 Jan 8;44(1):87-96.e5. doi: 10.1016/j.devcel.2017.12.012. Epub 2018 Jan 8. Dev Cell. 2018. PMID: 29316444 Free PMC article.
-
Organelle Optogenetics: Direct Manipulation of Intracellular Ca2+ Dynamics by Light.Front Neurosci. 2018 Aug 17;12:561. doi: 10.3389/fnins.2018.00561. eCollection 2018. Front Neurosci. 2018. PMID: 30174581 Free PMC article.
-
Visualization of Ca²+ signaling during embryonic skeletal muscle formation in vertebrates.Cold Spring Harb Perspect Biol. 2011 Feb 1;3(2):a004325. doi: 10.1101/cshperspect.a004325. Cold Spring Harb Perspect Biol. 2011. PMID: 21421918 Free PMC article. Review.
References
-
- Airey JA, Baring MD, Sutko JL. Ryanodine receptor protein is expressed during differentiation in the muscle cell lines BC3H1 and C2C12. Dev Biol. 1991;148:365–374. - PubMed
-
- Airey JA, Baring MD, Beck CF, Chelliah Y, Deerinck TJ, Ellisman MH, Houenou LJ, McKemy DD, Sutko JL, Talvenheimo J. Failure to make normal α ryanodine receptor is an early event associated with the crooked neck dwarf (cn) mutation in chicken. Dev Dynamics. 1993a;197:169–188. - PubMed
-
- Airey JA, Deerinck TJ, Ellisman MH, Houenou LJ, Ivanenko A, Kenyon JL, McKemy DD, Sutko JL. Crooked neck dwarf (cn) mutant chicken skeletal muscle cells in low density primary cultures fail to express normal alpha ryanodine receptor and exhibit a partial mutant phenotype. Dev Dyn. 1993b;197:189–202. - PubMed
-
- Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

