The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization
- PMID: 9628892
- PMCID: PMC2132798
- DOI: 10.1083/jcb.141.6.1357
The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization
Abstract
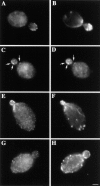
The budding yeast contains two type I myosins, Myo3p and Myo5p, with redundant functions. Deletion of both myosins results in growth defects, loss of actin polarity and polarized cell surface growth, and accumulation of intracellular membranes. Expression of myc-tagged Myo5p in myo3Delta myo5Delta cells fully restores wild-type characteristics. Myo5p is localized as punctate, cortical structures enriched at sites of polarized cell growth. We find that latrunculin-A-induced depolymerization of F-actin results in loss of Myo5p patches. Moreover, incubation of yeast cells at 37 degrees C results in transient depolarization of both Myo5p patches and the actin cytoskeleton. Mutant Myo5 proteins with deletions in nonmotor domains were expressed in myo3Delta myo5Delta cells and the resulting strains were analyzed for Myo5p function. Deletion of the tail homology 2 (TH2) domain, previously implicated in ATP-insensitive actin binding, has no detectable effect on Myo5p function. In contrast, myo3Delta myo5Delta cells expressing mutant Myo5 proteins with deletions of the src homology domain 3 (SH3) or both TH2 and SH3 domains display defects including Myo5p patch depolarization, actin disorganization, and phenotypes associated with actin dysfunction. These findings support a role for the SH3 domain in Myo5p localization and function in budding yeast. The proline-rich protein verprolin (Vrp1p) binds to the SH3 domain of Myo3p or Myo5p in two-hybrid tests, coimmunoprecipitates with Myo5p, and colocalizes with Myo5p. Immunolocalization of the myc-tagged SH3 domain of Myo5p reveals diffuse cytoplasmic staining. Thus, the SH3 domain of Myo5p contributes to but is not sufficient for localization of Myo5p either to patches or to sites of polarized cell growth. Consistent with this, Myo5p patches assemble but do not localize to sites of polarized cell surface growth in a VRP1 deletion mutant. Our studies support a multistep model for Myo5p targeting in yeast. The first step, assembly of Myo5p patches, is dependent upon F-actin, and the second step, polarization of actin patches, requiresVrp1p and the SH3 domain of Myo5p.
Figures






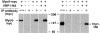
References
-
- Adams RJ, Pollard TD. Binding of myosin I to membrane lipids. Nature. 1989;340:565–568. - PubMed
-
- Adams AE, Botstein D, Drubin DG. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. . Nature. 1991;354:404–408. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

