Spindle assembly and mitosis without centrosomes in parthenogenetic Sciara embryos
- PMID: 9628894
- PMCID: PMC2132787
- DOI: 10.1083/jcb.141.6.1383
Spindle assembly and mitosis without centrosomes in parthenogenetic Sciara embryos
Abstract
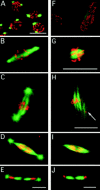
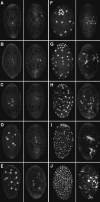
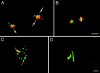
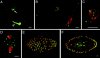
In Sciara, unfertilized embryos initiate parthenogenetic development without centrosomes. By comparing these embryos with normal fertilized embryos, spindle assembly and other microtubule-based events can be examined in the presence and absence of centrosomes. In both cases, functional mitotic spindles are formed that successfully proceed through anaphase and telophase, forming two daughter nuclei separated by a midbody. The spindles assembled without centrosomes are anastral, and it is likely that their microtubules are nucleated at or near the chromosomes. These spindles undergo anaphase B and successfully segregate sister chromosomes. However, without centrosomes the distance between the daughter nuclei in the next interphase is greatly reduced. This suggests that centrosomes are required to maintain nuclear spacing during the telophase to interphase transition. As in Drosophila, the initial embryonic divisions of Sciara are synchronous and syncytial. The nuclei in fertilized centrosome-bearing embryos maintain an even distribution as they divide and migrate to the cortex. In contrast, as division proceeds in embryos lacking centrosomes, nuclei collide and form large irregularly shaped nuclear clusters. These nuclei are not evenly distributed and never successfully migrate to the cortex. This phenotype is probably a direct result of a failure to form astral microtubules in parthenogenetic embryos lacking centrosomes. These results indicate that the primary function of centrosomes is to provide astral microtubules for proper nuclear spacing and migration during the syncytial divisions. Fertilized Sciara embryos produce a large population of centrosomes not associated with nuclei. These free centrosomes do not form spindles or migrate to the cortex and replicate at a significantly reduced rate. This suggests that the centrosome must maintain a proper association with the nucleus for migration and normal replication to occur.
Figures


References
-
- Aist JR, Bayles CJ, Tao W, Berns MW. Direct evidence for the existence, structural basis and function of astral forces during anaphase B in vivo. J Cell Sci. 1991;100:279–288. - PubMed
-
- Balczon R. The centrosome in animal cells and its functional homologs in plant and yeast cells. Int Rev Cytol. 1996;169:25–82. - PubMed
-
- Boleti H, Karsenti E, Vernos I. Xklp2, a novel Xenopuscentrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. - PubMed
-
- Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci. 1994;107:285–297. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

