OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner
- PMID: 9628896
- PMCID: PMC2132788
- DOI: 10.1083/jcb.141.6.1407
OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner
Abstract
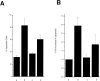
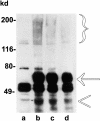
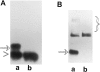
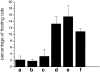
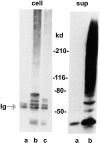
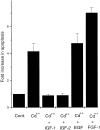
OCI-5/GPC3 is a member of the glypican family. Glypicans are heparan sulfate proteoglycans that are bound to the cell surface through a glycosyl-phosphatidylinositol anchor. It has recently been shown that the OCI-5/GPC3 gene is mutated in patients with the Simpson-Golabi-Behmel Syndrome (SGBS), an X-linked disorder characterized by pre- and postnatal overgrowth and various visceral and skeletal dysmorphisms. Some of these dysmorphisms could be the result of deficient growth inhibition or apoptosis in certain cell types during development. Here we present evidence indicating that OCI-5/GPC3 induces apoptosis in cell lines derived from mesothelioma (II14) and breast cancer (MCF-7). This induction, however, is cell line specific since it is not observed in NIH 3T3 fibroblasts or HT-29 colorectal tumor cells. We also show that the apoptosis-inducing activity in II14 and MCF-7 cells requires the anchoring of OCI-5/GPC3 to the cell membrane. The glycosaminoglycan chains, on the other hand, are not required. MCF-7 cells can be rescued from OCI-5/GPC3-induced cell death by insulin-like growth factor 2. This factor has been implicated in Beckwith-Wiedemann, an overgrowth syndrome that has many similarities with SGBS. The discovery that OCI-5/GPC3 is able to induce apoptosis in a cell line- specific manner provides an insight into the mechanism that, at least in part, is responsible for the phenotype of SGBS patients.
Figures







References
-
- Baker SJ, Markowitz S, Fearon ER, Willson JKV, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. - PubMed
-
- Bonneh-Barkay D, Shlissel M, Berman B, Shaoul E, Admon A, Vlodavsky I, Carey DJ, Asundi VK, Reich-Slotky R, Ron D. Identification of glypican as a dual modulator of the biological activity of fibroblast growth factors. J Biol Chem. 1997;272:12415–12421. - PubMed
-
- Cadwell, R.C., and G.F. Joyce. 1995. Mutagenesis by PCR. In PCR Primer, a Laboratory Manual. C.W. Dieffenbach and G.S. Dveksler, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 581–613.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

