Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion
- PMID: 9628900
- PMCID: PMC2132783
- DOI: 10.1083/jcb.141.6.1449
Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion
Abstract
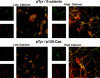
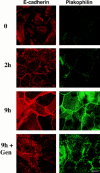
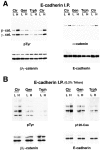
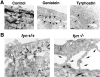
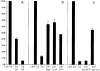
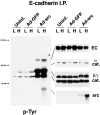
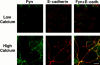
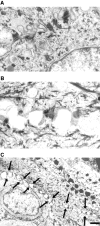
In their progression from the basal to upper differentiated layers of the epidermis, keratinocytes undergo significant structural changes, including establishment of close intercellular contacts. An important but so far unexplored question is how these early structural events are related to the biochemical pathways that trigger differentiation. We show here that beta-catenin, gamma-catenin/plakoglobin, and p120-Cas are all significantly tyrosine phosphorylated in primary mouse keratinocytes induced to differentiate by calcium, with a time course similar to that of cell junction formation. Together with these changes, there is an increased association of alpha-catenin and p120-Cas with E-cadherin, which is prevented by tyrosine kinase inhibition. Treatment of E-cadherin complexes with tyrosine-specific phosphatase reveals that the strength of alpha-catenin association is directly dependent on tyrosine phosphorylation. In parallel with the biochemical effects, tyrosine kinase inhibition suppresses formation of cell adhesive structures, and causes a significant reduction in adhesive strength of differentiating keratinocytes. The Fyn tyrosine kinase colocalizes with E-cadherin at the cell membrane in calcium-treated keratinocytes. Consistent with an involvement of this kinase, fyn-deficient keratinocytes have strongly decreased tyrosine phosphorylation levels of beta- and gamma-catenins and p120-Cas, and structural and functional abnormalities in cell adhesion similar to those caused by tyrosine kinase inhibitors. Whereas skin of fyn-/- mice appears normal, skin of mice with a disruption in both the fyn and src genes shows intrinsically reduced tyrosine phosphorylation of beta-catenin, strongly decreased p120-Cas levels, and important structural changes consistent with impaired keratinocyte cell adhesion. Thus, unlike what has been proposed for oncogene-transformed or mitogenically stimulated cells, in differentiating keratinocytes tyrosine phosphorylation plays a positive role in control of cell adhesion, and this regulatory function appears to be important both in vitro and in vivo.
Figures






References
-
- Aberle H, Schwartz H, Hoschuetzky H, Kemler R. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to α-catenin. J Biol Chem. 1996;271:1520–1526. - PubMed
-
- Amagai M, Fujimori T, Masunaga T, Shimizu H, Nishikawa T, Shimizu N, Takeichi M, Hashimoto T. Delayed assembly of desmosomes in keratinocytes with disrupted classic- cadherin-mediated cell adhesion by a dominant negative mutant. J Invest Dermatol. 1995;104:27–32. - PubMed
-
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. - PMC - PubMed
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

