Expression of Legionella pneumophila virulence traits in response to growth conditions
- PMID: 9632562
- PMCID: PMC108309
- DOI: 10.1128/IAI.66.7.3029-3034.1998
Expression of Legionella pneumophila virulence traits in response to growth conditions
Abstract
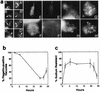
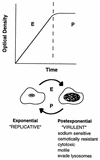
In nature, Legionella pneumophila replicates exclusively as an intracellular parasite of amoebae, but it also persists in the environment as a free-living microbe. Studies of how this opportunistic pathogen recognizes and responds to distinct extracellular and intracellular environments identified a link between the growth phase and expression of traits previously correlated with virulence. When cultured in broth, only post-exponential-phase L. pneumophila was sodium sensitive, cytotoxic, osmotically resistant, competent to evade macrophage lysosomes, infectious, and motile. Likewise, the L. pneumophila phenotype changed during growth in macrophages. During the intracellular replication period, this bacterium was sodium resistant and lacked flagella; concomitant with macrophage lysis, L. pneumophila became sodium sensitive and flagellated. Expression of the virulent phenotype was a response to starvation, since exponential-phase L. pneumophila became cytotoxic, sodium sensitive, and motile after incubation in broth from stationary-phase cultures, except when it was supplemented with amino acids. Together, these data indicate that while nutrients are plentiful, intracellular L. pneumophila organisms are dedicated to replication; when amino acids become limiting, the progeny express virulence factors to escape the spent host, to disperse and survive in the aquatic environment, and to reestablish a protected intracellular niche favorable for growth.
Figures

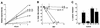
References
-
- Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

