In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli
- PMID: 9632579
- PMCID: PMC108326
- DOI: 10.1128/IAI.66.7.3149-3154.1998
In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli
Abstract
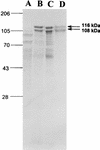
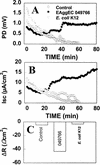
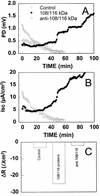
The pathogenic mechanisms of enteroaggregative Escherichia coli (EAggEC) infection are not fully elucidated. In this work we show that an ammonium sulfate precipitate of culture supernatant of EAggEC strain 049766 increased the potential difference (PD) and the short-circuit current (Isc) in rat jejunal preparations mounted in Ussing chambers. The precipitate contained two major proteins of 108 and 116 kDa, which were partially copurified by chromatography in DEAE-cellulose. This chromatographic fraction (peak I) increased jejunal PD and Isc in a dose-dependent manner, accompanied by a decrease in tissue electrical resistance. These effects were inhibited by incubation of peak I at 75 degreesC for 15 min or for 1 h with proteinase K at 37 degreesC. Rabbit polyclonal antibodies against peak I containing both the 108- and 116-kDa proteins inhibited the enterotoxic effect. Specific polyclonal antibodies raised against the 108-kDa but not against the 116-kDa protein inhibited the enterotoxic effect, suggesting that the 108-kDa protein is the active toxic species. Moreover, another EAggEC strain (065126) producing the 116-kDa protein but not the 108-kDa protein had no effect on rat jejunal mucosa in the Ussing chamber. The >100-kDa fraction derived from prototype EAggEC strain 042, which also expressed both 108- and 116-kDa proteins, also produced an enterotoxic effect on rat jejunal preparations in Ussing chambers; however, the same strain cured of its 65-MDa adherence plasmid did not. A subclone derived from the 65-MDa plasmid expressing the 108-kDa toxin (and not the 116-kDa protein) elicited rises in Isc. Tissue exposed to any preparation containing the 108-kDa toxin exhibited similar histopathologic changes, characterized by increased mucus release, exfoliation of cells, and development of crypt abscesses. Our data suggest that some EAggEC strains produce a ca. 108-kDa enterotoxin/cytotoxin which is encoded on the large virulence plasmid.
Figures
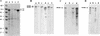


References
-
- Baudry B, Savarino S J, Vial P, Kaper J B, Levine M M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990;160:1249–1251. - PubMed
-
- Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

