Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli
- PMID: 9632580
- PMCID: PMC108327
- DOI: 10.1128/IAI.66.7.3155-3163.1998
Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli
Abstract
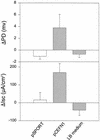
Enteroaggregative Escherichia coli (EAEC) is an emerging cause of diarrheal illness. Clinical data suggest that diarrhea caused by EAEC is predominantly secretory in nature, but the responsible enterotoxin has not been described. Work from our laboratories has implicated a ca. 108-kDa protein as a heat-labile enterotoxin and cytotoxin, as evidenced by rises in short-circuit current and falls in tissue resistance in rat jejunal tissue mounted in an Ussing chamber. Here we report the genetic cloning, sequencing, and characterization of this high-molecular-weight heat-labile toxin. The toxin (designated the plasmid-encoded toxin [Pet]) is encoded on the 65-MDa adherence-related plasmid of EAEC strain 042. Nucleotide sequence analysis suggests that the toxin is a member of the autotransporter class of proteins, characterized by the presence of a conserved C-terminal domain which forms a beta-barrel pore in the bacterial outer membrane and through which the mature protein is transported. The Pet toxin is highly homologous to the EspP protease of enterohemorrhagic E. coli and to EspC of enteropathogenic E. coli, an as yet cryptic protein. In addition to its potential role in EAEC infection, Pet represents the first enterotoxin within the autotransporter class of secreted proteins. We hypothesize that other closely related members of this class may also produce enterotoxic effects.
Figures



References
-
- Alting-Mees M A, Sorge J A, Short J M. pBluescriptII: multifunctional cloning and mapping vectors. Methods Enzymol. 1992;216:483–495. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989.
-
- Bachovchin W W, Plaut A G, Flentke G R, Lynch M, Kettner C A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990;265:3738–3743. - PubMed
-
- Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

