Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A
- PMID: 9632611
- PMCID: PMC108358
- DOI: 10.1128/IAI.66.7.3397-3402.1998
Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A
Abstract
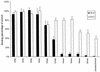
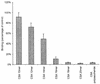
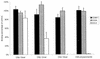
Adherence of parasite-infected erythrocytes (IEs) to the microvascular endothelium of various organs, a process known as sequestration, is a feature of Plasmodium falciparum malaria. This event is mediated by specific adhesive interactions between parasite proteins, expressed on the surface of IEs, and host molecules. P. falciparum IEs can bind to purified chondroitin sulfate A (CS-A), to the proteoglycan thrombomodulin through CS-A side chains, and to CS-A present on the surface of brain and lung endothelial cells and placental syncytiotrophoblasts. In order to identify structural characteristics of CS-A important for binding, oligosaccharide fragments ranging in size from 2 to 20 monosaccharide units were isolated from CS-A and CS-C, following controlled chondroitin lyase digestion, and used as competitive inhibitors of IE binding to immobilized ligands. Inhibition of binding to CS-A was highly dependent on molecular size: a CS-A tetradecasaccharide fraction was the minimum length able to almost completely inhibit binding. The effect was dose dependent and similar to that of the parent polysaccharide, and the same degree of inhibition was not found with the CS-C oligosaccharides. There was no effect on binding of IEs to other ligands, e.g., CD36 and intercellular adhesion molecule 1. Hexadeca- and octadecasaccharide fractions of CS-A were required for maximum inhibition of binding to thrombomodulin. Analyses of oligosaccharide fractions and polysaccharides by electrospray mass spectrometry and high-performance liquid chromatography suggest that the differences between the activities of CS-A and CS-C oligosaccharides can be attributed to differences in sulfate content and sulfation pattern and that iduronic acid is not involved in IE binding.
Figures

Similar articles
-
The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid.J Biol Chem. 2002 Jun 21;277(25):22438-46. doi: 10.1074/jbc.M111401200. Epub 2002 Apr 15. J Biol Chem. 2002. PMID: 11956186
-
Structural basis for binding of Plasmodium falciparum erythrocyte membrane protein 1 to chondroitin sulfate and placental tissue and the influence of protein polymorphisms on binding specificity.J Biol Chem. 2007 Aug 3;282(31):22426-36. doi: 10.1074/jbc.M700231200. Epub 2007 Jun 11. J Biol Chem. 2007. PMID: 17562715
-
Plasmodium falciparum-infected erythrocytes adhere to the proteoglycan thrombomodulin in static and flow-based systems.Exp Parasitol. 1997 May;86(1):8-18. doi: 10.1006/expr.1996.4142. Exp Parasitol. 1997. PMID: 9149236
-
Adherence of Plasmodium falciparum-infected erythrocytes to chondroitin 4-sulfate.Biosci Rep. 1999 Aug;19(4):261-71. doi: 10.1023/a:1020542206916. Biosci Rep. 1999. PMID: 10589991 Review.
-
Host receptors for malaria-infected erythrocytes.Am J Trop Med Hyg. 1990 Aug;43(2 Pt 2):6-14. doi: 10.4269/ajtmh.1990.43.6. Am J Trop Med Hyg. 1990. PMID: 1697144 Review.
Cited by
-
Evaluation of the antigenic diversity of placenta-binding Plasmodium falciparum variants and the antibody repertoire among pregnant women.Infect Immun. 2010 May;78(5):1963-78. doi: 10.1128/IAI.01365-09. Epub 2010 Feb 16. Infect Immun. 2010. PMID: 20160014 Free PMC article.
-
Regioselectively modified sulfated cellulose as prospective drug for treatment of malaria tropica.Glycoconj J. 2007 Jan;24(1):57-65. doi: 10.1007/s10719-006-9012-1. Glycoconj J. 2007. PMID: 17115275
-
Carrageenans inhibit the in vitro growth of Plasmodium falciparum and cytoadhesion to CD36.Parasitol Res. 2005 Oct;97(4):290-4. doi: 10.1007/s00436-005-1426-3. Epub 2005 Jul 13. Parasitol Res. 2005. PMID: 16012863
-
Progress in deciphering the information content of the 'glycome'--a crescendo in the closing years of the millennium.Glycoconj J. 2000 Jul-Sep;17(7-9):553-65. doi: 10.1023/a:1011022509500. Glycoconj J. 2000. PMID: 11421348
-
Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies.J Infect Dis. 2006 Mar 1;193(5):721-30. doi: 10.1086/500145. Epub 2006 Jan 30. J Infect Dis. 2006. PMID: 16453269 Free PMC article.
References
-
- Aikawa M, Iseki M, Barnwell J W, Taylor D, Oo M M, Howard R J. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990;43:30–37. - PubMed
-
- Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. - PubMed
-
- Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion molecule for Plasmodium falciparum. Nature. 1989;341:57–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

