Activation of c-myc gene expression by tumor-derived p53 mutants requires a discrete C-terminal domain
- PMID: 9632756
- PMCID: PMC108956
- DOI: 10.1128/MCB.18.7.3735
Activation of c-myc gene expression by tumor-derived p53 mutants requires a discrete C-terminal domain
Abstract
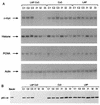
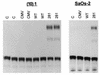
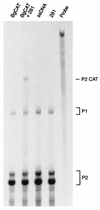
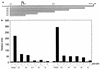
Mutation of the p53 tumor suppressor gene is the most common genetic alteration in human cancer, and tumors that express mutant p53 may be more aggressive and have a worse prognosis than p53-null cancers. Mutant p53 enhances tumorigenicity in the absence of a transdominant negative mechanism, and this tumor-promoting activity correlates with its ability to transactivate reporter genes in transient transfection assays. However, the mechanism by which mutant p53 functions in transactivation and its endogenous cellular targets that promote tumorigenicity are unknown. Here we report that (i) mutant p53 can regulate the expression of the endogenous c-myc gene and is a potent activator of the c-myc promoter; (ii) the region of mutant p53 responsiveness in the c-myc gene has been mapped to the 3' end of exon 1; (iii) the mutant p53 response region is position and orientation dependent and therefore does not function as an enhancer; and (iv) transactivation by mutant p53 requires the C terminus, which is not essential for wild-type p53 transactivation. These data suggest that it may be possible to selectively inhibit mutant p53 gain of function and consequently reduce the tumorigenic potential of cancer cells. A possible mechanism for transactivation of the c-myc gene by mutant p53 is proposed.
Figures


References
-
- Bentley D, Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986;321:702–706. - PubMed
-
- Chin K-V, Ueda K, Pastan I, Gottesman M M. Modulation of activity of the promoter of the human MDR1 gene by ras and p53. Science. 1992;255:459–462. - PubMed
-
- Cleveland J L, Dean M, Rosenberg N, Wang J W-Y, Rapp U R. Tyrosine kinase oncogenes abrogate interleukin-3 dependence of murine myeloid cells through signaling pathways involving c-myc: conditional regulation of c-myc transcription by temperature-sensitive v-abl. Mol Cell Biol. 1989;9:5685–5695. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
