Coactivation by OCA-B: definition of critical regions and synergism with general cofactors
- PMID: 9632764
- PMCID: PMC108964
- DOI: 10.1128/MCB.18.7.3803
Coactivation by OCA-B: definition of critical regions and synergism with general cofactors
Abstract
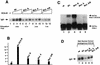
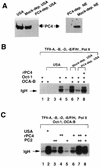
Molecular dissection of the B-cell-specific transcription coactivator OCA-B has revealed distinct regions important, respectively, for recruitment to immunoglobulin promoters through interaction with octamer-bound Oct-1 and for subsequent coactivator function. Further analysis of general coactivator requirements showed that selective removal of PC4 from the essential USA fraction severely impairs Oct-1 and OCA-B function in a cell-free system reconstituted with partially purified factors. Full activity can be restored by the combined action of recombinant PC4 and the PC4-depleted USA fraction, thus suggesting a joint requirement for PC4 and another, USA-derived component(s) for optimal function of Oct-1/OCA-B in the reconstituted system. Indeed, USA-derived PC2 was found to act synergistically with PC4 in reproducing the function of intact USA in the assay system. Consistent with the requirement for PC4 in the reconstituted system, OCA-B was found to interact directly with PC4. Surprisingly, however, removal of PC4 from the unfractionated nuclear extract has no detrimental effect on OCA-B/Oct-1-dependent transcription. These results lead to a general model for the synergistic function of activation domains in Oct-1 and OCA-B (mediated by the combined action of the multiple USA components) and, further, suggest a functional redundancy in general coactivators.
Figures



References
-
- Apone L M, Virbasius C A, Reese J C, Green M R. Yeast TAFII90 is required for cell-cycle progression through G2/M but not for general transcription activation. Genes Dev. 1996;10:2368–2380. - PubMed
-
- Björlund S, Kim Y-J. Mediator of transcriptional regulation. Trends Biochem Sci. 1996;21:335–337. - PubMed
-
- Cepek K L, Chasman D I, Sharp P A. Sequence-specific DNA binding of the B-cell-specific coactivator OCA-B. Genes Dev. 1996;10:2079–2088. - PubMed
-
- Cleary M A, Stern S, Tanaka M, Herr W. Differential positive control by Oct-1 and Oct-2: activation of a transcriptionally silent motif through Oct-1 and VP16 corecruitment. Genes Dev. 1993;7:72–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
