Differential requirements for alternative splicing and nuclear export functions of equine infectious anemia virus Rev protein
- PMID: 9632773
- PMCID: PMC108973
- DOI: 10.1128/MCB.18.7.3889
Differential requirements for alternative splicing and nuclear export functions of equine infectious anemia virus Rev protein
Abstract
The Rev protein of equine infectious anemia virus (ERev) exports unspliced and partially spliced viral RNAs from the nucleus. Like several cellular proteins, ERev regulates its own mRNA by mediating an alternative splicing event. To determine the requirements for these functions, we have identified ERev mutants that affect RNA export or both export and alternative splicing. Mutants were further characterized for subcellular localization, nuclear-cytoplasmic shuttling, and multimerization. None of the nuclear export signal (NES) mutants are defective for alternative splicing. Furthermore, the NES of ERev is similar in composition but distinct in spacing from other leucine-rich NESs. Basic residues at the C terminus of ERev are involved in nuclear localization, and disruption of the C-terminal residues affects both functions of ERev. ERev forms multimers, and no mutation disrupts this activity. In two mutants with substitutions of charged residues in the middle of ERev, RNA export is affected. One of these mutants is also defective for ERev-mediated alternative splicing but is identical to wild-type ERev in its localization, shuttling, and multimerization. Together, these results demonstrate that the two functions of ERev both require nuclear import and at least one other common activity, but RNA export can be separated from alternative splicing based on its requirement for a functional NES.
Figures




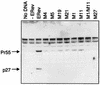



Similar articles
-
Binding sites for Rev and ASF/SF2 map to a 55-nucleotide purine-rich exonic element in equine infectious anemia virus RNA.J Biol Chem. 2001 Jun 1;276(22):18960-7. doi: 10.1074/jbc.M008996200. Epub 2001 Mar 16. J Biol Chem. 2001. PMID: 11278454
-
Binding of equine infectious anemia virus rev to an exon splicing enhancer mediates alternative splicing and nuclear export of viral mRNAs.Mol Cell Biol. 2000 May;20(10):3550-7. doi: 10.1128/MCB.20.10.3550-3557.2000. Mol Cell Biol. 2000. PMID: 10779344 Free PMC article.
-
Nuclear transport of human immunodeficiency virus type 1, visna virus, and equine infectious anemia virus Rev proteins: identification of a family of transferable nuclear export signals.J Virol. 1996 Apr;70(4):2350-9. doi: 10.1128/JVI.70.4.2350-2359.1996. J Virol. 1996. PMID: 8642662 Free PMC article.
-
The HIV-1 Rev protein.Annu Rev Microbiol. 1998;52:491-532. doi: 10.1146/annurev.micro.52.1.491. Annu Rev Microbiol. 1998. PMID: 9891806 Review.
-
Nucleocytoplasmic RNA transport in retroviral replication.Results Probl Cell Differ. 2001;34:197-217. doi: 10.1007/978-3-540-40025-7_12. Results Probl Cell Differ. 2001. PMID: 11288676 Review.
Cited by
-
Identifying interaction sites in "recalcitrant" proteins: predicted protein and RNA binding sites in rev proteins of HIV-1 and EIAV agree with experimental data.Pac Symp Biocomput. 2006:415-26. Pac Symp Biocomput. 2006. PMID: 17094257 Free PMC article.
-
Altered expression of tyrosine kinases of the Src and Syk families in human T-cell leukemia virus type 1-infected T-cell lines.J Virol. 1999 May;73(5):3709-17. doi: 10.1128/JVI.73.5.3709-3717.1999. J Virol. 1999. PMID: 10196263 Free PMC article.
-
Rev variation during persistent lentivirus infection.Viruses. 2011 Jan;3(1):1-11. doi: 10.3390/v3010001. Epub 2011 Jan 11. Viruses. 2011. PMID: 21994723 Free PMC article. Review.
-
Equine lentivirus counteracts SAMHD1 restriction by Rev-mediated degradation of SAMHD1 via the BECN1-dependent lysosomal pathway.Autophagy. 2021 Oct;17(10):2800-2817. doi: 10.1080/15548627.2020.1846301. Epub 2020 Nov 24. Autophagy. 2021. PMID: 33172327 Free PMC article.
-
Identification of a Novel Post-transcriptional Transactivator from the Equine Infectious Anemia Virus.J Virol. 2022 Dec 21;96(24):e0121022. doi: 10.1128/jvi.01210-22. Epub 2022 Nov 30. J Virol. 2022. PMID: 36448796 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
