Simian virus 40 large T antigen stabilizes the TATA-binding protein-TFIIA complex on the TATA element
- PMID: 9632777
- PMCID: PMC108977
- DOI: 10.1128/MCB.18.7.3926
Simian virus 40 large T antigen stabilizes the TATA-binding protein-TFIIA complex on the TATA element
Abstract
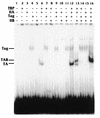
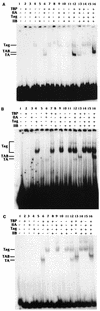
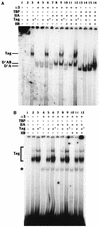
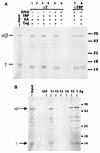
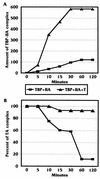
Large T antigen (T antigen), the early gene product of simian virus 40 (SV40), is a potent transcriptional activator of both cellular and viral genes. Recently we have shown that T antigen is tightly associated with TFIID and, in this position, performs a TATA-binding protein (TBP)-associated factor (TAF)-like function. Based on this observation, we asked whether T antigen affected steps in preinitiation complex assembly. Using purified components in in vitro complex assembly assays, we found that T antigen specifically enhances the formation of the TBP-TFIIA complex on the TATA element. T antigen accomplishes this by increasing the rate of formation of the TBP-TFIIA complex on the TATA element and by stabilizing the complexes after they are formed on the promoter. In addition, DNA immunoprecipitation experiments indicate that T antigen is associated with the stabilized TBP-TFIIA complexes bound to the DNA. In this regard, it has previously been shown that T antigen interacts with TBP; in the present study, we show that T antigen also interacts with TFIIA in vitro. In testing the ability of T antigen to stabilize the TBP-TFIIA complex, we found that stabilization is highly sensitive to the specific sequence context of the TATA element. Previous studies showed that T antigen could activate simple promoters containing the TATA elements from the hsp70 and c-fos gene promoters but failed to significantly activate similar promoters containing the TATA elements from the promoters of the SV40 early and adenovirus E2a genes. We find that the ability to stabilize the TBP-TFIIA complex on the hsp70 and c-fos TATA elements, and not on the SV40 early and E2A TATA elements, correlates with the ability or inability to activate promoters containing these TATA elements.
Figures




Similar articles
-
Simian virus 40 large T antigen interacts with human TFIIB-related factor and small nuclear RNA-activating protein complex for transcriptional activation of TATA-containing polymerase III promoters.Mol Cell Biol. 1998 Mar;18(3):1331-8. doi: 10.1128/MCB.18.3.1331. Mol Cell Biol. 1998. PMID: 9488448 Free PMC article.
-
Stabilized binding of TBP to the TATA box of herpes simplex virus type 1 early (tk) and late (gC) promoters by TFIIA and ICP4.J Virol. 2008 Apr;82(7):3546-54. doi: 10.1128/JVI.02560-07. Epub 2008 Jan 23. J Virol. 2008. PMID: 18216093 Free PMC article.
-
Virtually unidirectional binding of TBP to the AdMLP TATA box within the quaternary complex with TFIIA and TFIIB.Chem Biol. 2000 Aug;7(8):601-10. doi: 10.1016/s1074-5521(00)00009-0. Chem Biol. 2000. PMID: 11048951
-
Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation.Gene. 2022 Jul 30;833:146581. doi: 10.1016/j.gene.2022.146581. Epub 2022 May 18. Gene. 2022. PMID: 35597524 Review.
-
Structural insights into assembly of transcription preinitiation complex.Curr Opin Struct Biol. 2022 Aug;75:102404. doi: 10.1016/j.sbi.2022.102404. Epub 2022 Jun 11. Curr Opin Struct Biol. 2022. PMID: 35700575 Review.
Cited by
-
Tat gets the "green" light on transcription initiation.Retrovirology. 2005 Nov 9;2:69. doi: 10.1186/1742-4690-2-69. Retrovirology. 2005. PMID: 16280076 Free PMC article. Review.
-
A kinase activity associated with simian virus 40 large T antigen phosphorylates upstream binding factor (UBF) and promotes formation of a stable initiation complex between UBF and SL1.Mol Cell Biol. 1999 Apr;19(4):2791-802. doi: 10.1128/MCB.19.4.2791. Mol Cell Biol. 1999. PMID: 10082545 Free PMC article.
-
Proteomics Analysis of the Polyomavirus DNA Replication Initiation Complex Reveals Novel Functional Phosphorylated Residues and Associated Proteins.Int J Mol Sci. 2024 Apr 21;25(8):4540. doi: 10.3390/ijms25084540. Int J Mol Sci. 2024. PMID: 38674125 Free PMC article.
-
Interaction of the transcription factor TFIID with simian virus 40 (SV40) large T antigen interferes with replication of SV40 DNA in vitro.J Virol. 1999 Feb;73(2):1099-107. doi: 10.1128/JVI.73.2.1099-1107.1999. J Virol. 1999. PMID: 9882311 Free PMC article.
-
Transcriptional activation in yeast cells lacking transcription factor IIA.Genetics. 1999 Dec;153(4):1573-81. doi: 10.1093/genetics/153.4.1573. Genetics. 1999. PMID: 10581267 Free PMC article.
References
-
- Beard P, Bruggmann H. Control of transcription in vitro from simian virus 40 promoters by proteins from viral minichromosomes. Curr Top Microbiol Immunol. 1989;144:47–54. - PubMed
-
- Bryant G, Martel L, Burley S K, Berk A J. Radical mutations reveal TATA-box binding protein surfaces required for activated transcription in vivo. Genes Dev. 1996;10:2491–2504. - PubMed
-
- Buratowski S, Hahn S, Guarente L, Sharp P A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989;56:549–561. - PubMed
-
- Buratowski S. Multiple TATA-binding factors come back into style. Cell. 1997;91:13–15. - PubMed
-
- Chen J L, Attardi L D, Verrijzer C P, Yokomori K, Tjian R. Assembly of recombinant TFIID reveals different coactivator requirements for distinct transcriptional activators. Cell. 1994;79:93–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
