Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation
- PMID: 9632781
- PMCID: PMC108981
- DOI: 10.1128/MCB.18.7.3966
Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation
Abstract
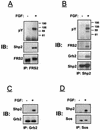
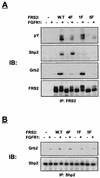
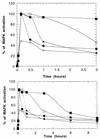
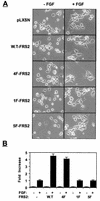
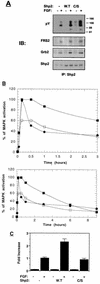
FRS2 is a lipid-anchored docking protein that plays an important role in linking fibroblast growth factor (FGF) and nerve growth factor receptors with the Ras/mitogen-activated protein (MAP) kinase signaling pathway. In this report, we demonstrate that FRS2 forms a complex with the N-terminal SH2 domain of the protein tyrosine phosphatase Shp2 in response to FGF stimulation. FGF stimulation induces tyrosine phosphorylation of Shp2, leading to the formation of a complex containing Grb2 and Sos1 molecules. In addition, a mutant FRS2 deficient in both Grb2 and Shp2 binding induces a weak and transient MAP kinase response and fails to induce PC12 cell differentiation in response to FGF stimulation. Furthermore, FGF is unable to induce differentiation of PC12 cells expressing an FRS2 point mutant deficient in Shp2 binding. Finally, we demonstrate that the catalytic activity of Shp2 is essential for sustained activation of MAP kinase and for potentiation of FGF-induced PC12 cell differentiation. These experiments demonstrate that FRS2 recruits Grb2 molecules both directly and indirectly via complex formation with Shp2 and that Shp2 plays an important role in FGF-induced PC12 cell differentiation.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
