RNA binding activity of heterodimeric splicing factor U2AF: at least one RS domain is required for high-affinity binding
- PMID: 9632785
- PMCID: PMC108985
- DOI: 10.1128/MCB.18.7.4004
RNA binding activity of heterodimeric splicing factor U2AF: at least one RS domain is required for high-affinity binding
Abstract
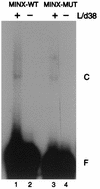
The pre-mRNA splicing factor U2AF (U2 small nuclear ribonucleoprotein particle [snRNP] auxiliary factor) plays a critical role in 3' splice site selection. U2AF binds site specifically to the intron pyrimidine tract between the branchpoint and the 3' splice site and targets U2 snRNP to the branch site at an early step in spliceosome assembly. Human U2AF is a heterodimer composed of large (hU2AF65) and small (hU2AF35) subunits. hU2AF65 contains an arginine-serine-rich (RS) domain and three RNA recognition motifs (RRMs). hU2AF35 has a degenerate RRM and a carboxyl-terminal RS domain. Genetic studies have recently shown that the RS domains on the Drosophila U2AF subunit homologs are each inessential and might have redundant functions in vivo. The site-specific pyrimidine tract binding activity of the U2AF heterodimer has previously been assigned to hU2AF65. While the requirement for the three RRMs on hU2AF65 is firmly established, a role for the large-subunit RS domain in RNA binding remains unresolved. We have analyzed the RNA binding activity of the U2AF heterodimer in vitro. When the Drosophila small-subunit homolog (dU2AF38) was complexed with the large-subunit (dU2AF50) pyrimidine tract, RNA binding activity increased 20-fold over that of free dU2AF50. We detected a similar increase in RNA binding activity when we compared the human U2AF heterodimer and hU2AF65. Surprisingly, the RS domain on dU2AF38 was necessary for the increased binding activity of the dU2AF heterodimer. In addition, removal of the RS domain from the Drosophila large-subunit monomer (dU2AF50DeltaRS) severely impaired its binding activity. However, if the dU2AF38 RS domain was supplied in a complex with dU2AF50DeltaRS, high-affinity binding was restored. These results suggest that the presence of one RS domain of U2AF, on either the large or small subunit, promotes high-affinity pyrimidine tract RNA binding activity, consistent with redundant roles for the U2AF RS domains in vivo.
Figures



Similar articles
-
U2AF homology motifs: protein recognition in the RRM world.Genes Dev. 2004 Jul 1;18(13):1513-26. doi: 10.1101/gad.1206204. Genes Dev. 2004. PMID: 15231733 Free PMC article. Review.
-
Molecular genetic analysis of the heterodimeric splicing factor U2AF: the RS domain on either the large or small Drosophila subunit is dispensable in vivo.Genes Dev. 1998 Apr 1;12(7):1010-21. doi: 10.1101/gad.12.7.1010. Genes Dev. 1998. PMID: 9531538 Free PMC article.
-
Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects.Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10333-7. doi: 10.1073/pnas.93.19.10333. Proc Natl Acad Sci U S A. 1996. PMID: 8816800 Free PMC article.
-
Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo.Mol Cell Biol. 1998 Apr;18(4):1765-73. doi: 10.1128/MCB.18.4.1765. Mol Cell Biol. 1998. PMID: 9528748 Free PMC article.
-
A protein interaction domain contacts RNA in the prespliceosome.Mol Cell. 2004 Feb 13;13(3):302-4. doi: 10.1016/s1097-2765(04)00055-3. Mol Cell. 2004. PMID: 14967137 Review.
Cited by
-
U2AF1 mutations alter sequence specificity of pre-mRNA binding and splicing.Leukemia. 2015 Apr;29(4):909-17. doi: 10.1038/leu.2014.303. Epub 2014 Oct 14. Leukemia. 2015. PMID: 25311244 Free PMC article.
-
U2AF homology motifs: protein recognition in the RRM world.Genes Dev. 2004 Jul 1;18(13):1513-26. doi: 10.1101/gad.1206204. Genes Dev. 2004. PMID: 15231733 Free PMC article. Review.
-
In vivo requirement of the small subunit of U2AF for recognition of a weak 3' splice site.Mol Cell Biol. 2006 Nov;26(21):8183-90. doi: 10.1128/MCB.00350-06. Epub 2006 Aug 28. Mol Cell Biol. 2006. PMID: 16940179 Free PMC article.
-
RNA-protein interactions that regulate pre-mRNA splicing.Gene Expr. 2002;10(1-2):79-92. Gene Expr. 2002. PMID: 11868989 Free PMC article. Review.
-
An Unusual U2AF2 Inhibits Splicing and Attenuates the Virulence of the Human Protozoan Parasite Entamoeba histolytica.Front Cell Infect Microbiol. 2022 Jun 17;12:888428. doi: 10.3389/fcimb.2022.888428. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782149 Free PMC article.
References
-
- Blumenthal, T. Personal communication.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
