Role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor
- PMID: 9632792
- PMCID: PMC108992
- DOI: 10.1128/MCB.18.7.4079
Role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor
Abstract
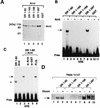
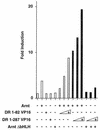
The dioxin receptor is a ligand-regulated transcription factor that mediates signal transduction by dioxin and related environmental pollutants. The receptor belongs to the basic helix-loop-helix (bHLH)-Per-Arnt-Sim (PAS) family of factors, which, in addition to the bHLH motif, contain a PAS region of homology. Upon activation, the dioxin receptor dimerizes with the bHLH-PAS factor Arnt, enabling the receptor to recognize xenobiotic response elements in the vicinity of target genes. We have studied the role of the PAS domain in dimerization and DNA binding specificity of the dioxin receptor and Arnt by monitoring the abilities of the individual bHLH domains and different bHLH-PAS fragments to dimerize and bind DNA in vitro and recognize target genes in vivo. The minimal bHLH domain of the dioxin receptor formed homodimeric complexes, heterodimerized with full-length Arnt, and together with Arnt was sufficient for recognition of target DNA in vitro and in vivo. In a similar fashion, only the bHLH domain of Arnt was necessary for DNA binding specificity in the presence of the dioxin receptor bHLH domain. Moreover, the bHLH domain of the dioxin receptor displayed a broad dimerization potential, as manifested by complex formation with, e.g. , the unrelated bHLH-Zip transcription factor USF. In contrast, a construct spanning the dioxin receptor bHLH domain and an N-terminal portion of the PAS domain failed to form homodimers and was capable of dimerizing only with Arnt. Thus, the PAS domain is essential to confer dimerization specificity of the dioxin receptor.
Figures






Similar articles
-
Protein-protein interaction via PAS domains: role of the PAS domain in positive and negative regulation of the bHLH/PAS dioxin receptor-Arnt transcription factor complex.EMBO J. 1995 Jul 17;14(14):3528-39. doi: 10.1002/j.1460-2075.1995.tb07359.x. EMBO J. 1995. PMID: 7628454 Free PMC article.
-
Heterodimers of bHLH-PAS protein fragments derived from AhR, AhRR, and Arnt prepared by co-expression in Escherichia coli: characterization of their DNA binding activity and preparation of a DNA complex.J Biochem. 2003 Jul;134(1):83-90. doi: 10.1093/jb/mvg115. J Biochem. 2003. PMID: 12944374
-
Identification of transactivation and repression functions of the dioxin receptor and its basic helix-loop-helix/PAS partner factor Arnt: inducible versus constitutive modes of regulation.Mol Cell Biol. 1994 Dec;14(12):8343-55. doi: 10.1128/mcb.14.12.8343-8355.1994. Mol Cell Biol. 1994. PMID: 7969169 Free PMC article.
-
Aryl hydrocarbon receptor-mediated signal transduction.Crit Rev Toxicol. 1997 Mar;27(2):109-34. doi: 10.3109/10408449709021615. Crit Rev Toxicol. 1997. PMID: 9099515 Review.
-
The mammalian basic helix-loop-helix/PAS family of transcriptional regulators.Int J Biochem Cell Biol. 2004 Feb;36(2):189-204. doi: 10.1016/s1357-2725(03)00211-5. Int J Biochem Cell Biol. 2004. PMID: 14643885 Review.
Cited by
-
Structure and dimerization properties of the aryl hydrocarbon receptor PAS-A domain.Mol Cell Biol. 2013 Nov;33(21):4346-56. doi: 10.1128/MCB.00698-13. Epub 2013 Sep 3. Mol Cell Biol. 2013. PMID: 24001774 Free PMC article.
-
PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora.Mol Cell Biol. 2002 Jan;22(2):517-24. doi: 10.1128/MCB.22.2.517-524.2002. Mol Cell Biol. 2002. PMID: 11756547 Free PMC article.
-
Domain characteristics, classification and expression profiles in response to various abiotic stresses of four HD-Zip subfamilies in tea plant.BMC Plant Biol. 2025 Jun 3;25(1):751. doi: 10.1186/s12870-025-06619-2. BMC Plant Biol. 2025. PMID: 40461974 Free PMC article.
-
Regulation of coronafacoyl phytotoxin production by the PAS-LuxR family regulator CfaR in the common scab pathogen Streptomyces scabies.PLoS One. 2015 Mar 31;10(3):e0122450. doi: 10.1371/journal.pone.0122450. eCollection 2015. PLoS One. 2015. PMID: 25826255 Free PMC article.
-
MK-6482 as a potential treatment for von Hippel-Lindau disease-associated clear cell renal cell carcinoma.Expert Opin Investig Drugs. 2021 May;30(5):495-504. doi: 10.1080/13543784.2021.1925248. Epub 2021 May 20. Expert Opin Investig Drugs. 2021. PMID: 33945366 Free PMC article. Review.
References
-
- Amati B, Dalton S, Brooks M W, Littlewood T D, Evan G I, Land H. Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature. 1992;359:423–426. - PubMed
-
- Antonsson C, Arulampalam V, Whitelaw M L, Pettersson S, Poellinger L. Constitutive function of the basic helix-loop-helix PAS factor Arnt—regulation of target promoters via the E-box motif. J Biol Chem. 1995;270:13968–13972. - PubMed
-
- Beckmann H, Kadesch T. The leucine zipper of TFE3 dictates helix-loop-helix dimerization specificity. Genes Dev. 1991;5:1057–1066. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
