Involvement of prolonged ras activation in thrombopoietin-induced megakaryocytic differentiation of a human factor-dependent hematopoietic cell line
- PMID: 9632812
- PMCID: PMC109012
- DOI: 10.1128/MCB.18.7.4282
Involvement of prolonged ras activation in thrombopoietin-induced megakaryocytic differentiation of a human factor-dependent hematopoietic cell line
Abstract
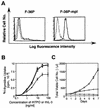
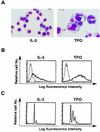
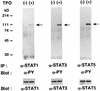
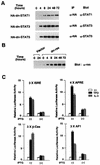
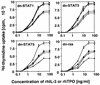
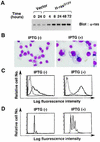
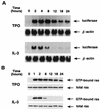
Thrombopoietin (TPO) is a hematopoietic growth factor that plays fundamental roles is both megakaryopoiesis and thrombopoiesis through binding to its receptor, c-mpl. Although TPO has been shown to activate various types of intracellular signaling molecules, such as the Janus family of protein tyrosine kinases, signal transducers and activators of transcription (STATs), and ras, the precise mechanisms underlying TPO-induced proliferation and differentiation remain unknown. In an effort to clarify the mechanisms of TPO-induced proliferation and differentiation, c-mpl was introduced into F-36P, a human interleukin-3 (IL-3)-dependent erythroleukemia cell line, and the effects of TPO on the c-mpl-transfected F-36P (F-36P-mpl) cells were investigated. F-36P-mpl cells were found to proliferate and differentiate at a high rate into mature megakaryocytes in response to TPO. Dominant-negative (dn) forms of STAT1, STAT3, STAT5, and ras were inducibly expressed in F-36P-mpl cells, and their effects on TPO-induced proliferation and megakaryocytic differentiation were analyzed. Among these dn molecules, both dn ras and dn STAT5 reduced TPO- or IL-3-induced proliferation of F-36P-mpl cells by approximately 30%, and only dn ras could inhibit TPO-induced megakaryocytic differentiation. In accord with this result, overexpression of activated ras (H-rasG12V) for 5 days led to megakaryocytic differentiation of F-36P-mpl cells. In a time course analysis on H-rasG12V-induced differentiation, activation of the ras pathway for 24 to 28 h was required and sufficient to induce megakaryocytic differentiation. Consistent with this result, the treatment of F-36P-mpl cells with TPO was able to induce prolonged activation of ras for more than 24 h, whereas IL-3 had only a transient effect. These results suggest that prolonged ras activation may be involved in TPO-induced megakaryocytic differentiation.
Figures



References
-
- Chiba S, Takaku F, Tange T, Shibuya K, Misawa C, Sasaki K, Miyagawa K, Yazaki Y, Hirai H. Establishment and erythroid differentiation of a cytokine-dependent human leukemia cell line F-36P: a parental line requiring granulocyte-macrophage colony-stimulating factor or interleukin-3, and a subline requiring erythropoietin. Blood. 1991;78:2261–2268. - PubMed
-
- de Rooij J, Bos J L. Minimal ras-binding domain of raf1 can be used as an activation-specific probe for ras. Oncogene. 1997;14:623–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
