Differential withdrawal of retinal axons induced by a secreted factor
- PMID: 9634566
- PMCID: PMC6792549
- DOI: 10.1523/JNEUROSCI.18-13-05008.1998
Differential withdrawal of retinal axons induced by a secreted factor
Abstract
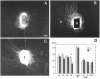
To understand the development of the topographic map in the chick retinotectal projection, we studied the long-term interactions between retinal axons and tectal cell processes using a novel coculture system, the ryomen chamber. Both nasal and temporal retinal axons initially grew equally well on a substrate consisting of posterior tectal cell processes; however, subsequently most temporal axons withdrew from this surface, whereas most nasal axons did not. Experiments using conditioned media indicate that posterior tectal cells induced withdrawal of the temporal axons by secreting a soluble factor. This withdrawal seems to be distinct from the immediate repulsive effect of ephrin-A2 (ELF-1) and ephrin-A5 (RAGS) seen in the stripe assay because (1) the withdrawal-inducing factor was diffusible, whereas ephrin-A2 and -A5 are membrane-bound, and (2) the withdrawal-inducing factor appeared later in development than ephrin-A2 and -A5. Furthermore, sensitivity to the withdrawal-inducing factor decreased continuously from the temporal to nasal retina. These results suggest that target cell-induced axonal withdrawal may be involved during a late stage of the development of the retinotectal map.
Figures

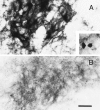
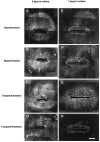
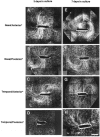
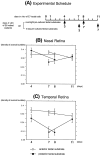

References
-
- Carri NG, Ebendal T. Target-field specificity in the induction of retinal neurite outgrowth. Brain Res. 1987;428:83–90. - PubMed
-
- Cheng H-J, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. - PubMed
-
- Cheng H-J, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. - PubMed
-
- Constantine-Paton M, Blum AS, Mendez Otero R, Barnstable CJ. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature. 1986;324:459–462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
