A synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 recognizes the wild-type fusion peptide in the membrane and inhibits HIV-1 envelope glycoprotein-mediated cell fusion
- PMID: 9636141
- PMCID: PMC22592
- DOI: 10.1073/pnas.95.13.7287
A synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 recognizes the wild-type fusion peptide in the membrane and inhibits HIV-1 envelope glycoprotein-mediated cell fusion
Abstract
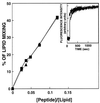
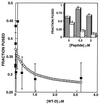
Recent studies demonstrated that a synthetic fusion peptide of HIV-1 self-associates in phospholipid membranes and inhibits HIV-1 envelope glycoprotein-mediated cell fusion, presumably by interacting with the N-terminal domain of gp41 and forming inactive heteroaggregates [Kliger, Y., Aharoni, A., Rapaport, D., Jones, P., Blumenthal, R. & Shai, Y. (1997) J. Biol. Chem. 272, 13496-13505]. Here, we show that a synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 (D-WT) of HIV-1 associates with its enantiomeric wild-type fusion (WT) peptide in the membrane and inhibits cell fusion mediated by the HIV-1 envelope glycoprotein. D-WT does not inhibit cell fusion mediated by the HIV-2 envelope glycoprotein. WT and D-WT are equally potent in inducing membrane fusion. D-WT peptide but not WT peptide is resistant to proteolytic digestion. Structural analysis showed that the CD spectra of D-WT in trifluoroethanol/water is a mirror image of that of WT, and attenuated total reflectance-fourier transform infrared spectroscopy revealed similar structures and orientation for the two enantiomers in the membrane. The results reveal that the chirality of the synthetic peptide corresponding to the HIV-1 gp41 N-terminal sequence does not play a role in liposome fusion and that the peptides' chirality is not necessarily required for peptide-peptide interaction within the membrane environment. Furthermore, studies along these lines may provide criteria to design protease-resistant therapeutic agents against HIV and other viruses.
Figures



Similar articles
-
Fusion peptides derived from the HIV type 1 glycoprotein 41 associate within phospholipid membranes and inhibit cell-cell Fusion. Structure-function study.J Biol Chem. 1997 May 23;272(21):13496-505. doi: 10.1074/jbc.272.21.13496. J Biol Chem. 1997. PMID: 9153194
-
Mode of action of an antiviral peptide from HIV-1. Inhibition at a post-lipid mixing stage.J Biol Chem. 2001 Jan 12;276(2):1391-7. doi: 10.1074/jbc.M004113200. J Biol Chem. 2001. PMID: 11027678
-
Effect of nonpolar substitutions of the conserved Phe11 in the fusion peptide of HIV-1 gp41 on its function, structure, and organization in membranes.Biochemistry. 1999 Aug 31;38(35):11359-71. doi: 10.1021/bi990232e. Biochemistry. 1999. PMID: 10471286
-
Peptide and non-peptide HIV fusion inhibitors.Curr Pharm Des. 2002;8(8):563-80. doi: 10.2174/1381612024607180. Curr Pharm Des. 2002. PMID: 11945159 Review.
-
Role of HIV Gp41 mediated fusion/hemifusion in bystander apoptosis.Cell Mol Life Sci. 2008 Oct;65(20):3134-44. doi: 10.1007/s00018-008-8147-6. Cell Mol Life Sci. 2008. PMID: 18500445 Free PMC article. Review.
Cited by
-
The three lives of viral fusion peptides.Chem Phys Lipids. 2014 Jul;181:40-55. doi: 10.1016/j.chemphyslip.2014.03.003. Epub 2014 Apr 2. Chem Phys Lipids. 2014. PMID: 24704587 Free PMC article. Review.
-
Targeting HIV-1 gp41-induced fusion and pathogenesis for anti-viral therapy.Curr Top Med Chem. 2011 Dec;11(24):2947-58. doi: 10.2174/156802611798808479. Curr Top Med Chem. 2011. PMID: 22044225 Free PMC article. Review.
-
Structural characterization of the SARS-coronavirus spike S fusion protein core.J Biol Chem. 2004 May 14;279(20):20836-49. doi: 10.1074/jbc.M400759200. Epub 2004 Mar 2. J Biol Chem. 2004. PMID: 14996844 Free PMC article.
-
A critical evaluation of the conformational requirements of fusogenic peptides in membranes.Eur Biophys J. 2007 Apr;36(4-5):405-13. doi: 10.1007/s00249-006-0106-2. Epub 2006 Nov 7. Eur Biophys J. 2007. PMID: 17089152
-
Mirror-image streptavidin with specific binding to L-biotin, the unnatural enantiomer.Sci Rep. 2022 Jun 10;12(1):9568. doi: 10.1038/s41598-022-13763-4. Sci Rep. 2022. PMID: 35688934 Free PMC article.
References
-
- White J M. Science. 1992;258:917–924. - PubMed
-
- Veronese F D, DeVico A L, Copeland T D, Oroszlan S, Gallo R C, Sarngadharan M G. Science. 1985;229:1402–1405. - PubMed
-
- Lasky L A, Nakamura G, Smith D H, Fennie C, Shimasaki C, Patzer E, Berman P, Gregory T, Capon D J. Cell. 1987;50:975–985. - PubMed
-
- Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Science. 1987;237:1351–1355. - PubMed
-
- Gallaher W R. Cell. 1987;50:327–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

