A synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 recognizes the wild-type fusion peptide in the membrane and inhibits HIV-1 envelope glycoprotein-mediated cell fusion
- PMID: 9636141
- PMCID: PMC22592
- DOI: 10.1073/pnas.95.13.7287
A synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 recognizes the wild-type fusion peptide in the membrane and inhibits HIV-1 envelope glycoprotein-mediated cell fusion
Abstract
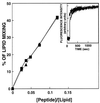
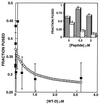
Recent studies demonstrated that a synthetic fusion peptide of HIV-1 self-associates in phospholipid membranes and inhibits HIV-1 envelope glycoprotein-mediated cell fusion, presumably by interacting with the N-terminal domain of gp41 and forming inactive heteroaggregates [Kliger, Y., Aharoni, A., Rapaport, D., Jones, P., Blumenthal, R. & Shai, Y. (1997) J. Biol. Chem. 272, 13496-13505]. Here, we show that a synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 (D-WT) of HIV-1 associates with its enantiomeric wild-type fusion (WT) peptide in the membrane and inhibits cell fusion mediated by the HIV-1 envelope glycoprotein. D-WT does not inhibit cell fusion mediated by the HIV-2 envelope glycoprotein. WT and D-WT are equally potent in inducing membrane fusion. D-WT peptide but not WT peptide is resistant to proteolytic digestion. Structural analysis showed that the CD spectra of D-WT in trifluoroethanol/water is a mirror image of that of WT, and attenuated total reflectance-fourier transform infrared spectroscopy revealed similar structures and orientation for the two enantiomers in the membrane. The results reveal that the chirality of the synthetic peptide corresponding to the HIV-1 gp41 N-terminal sequence does not play a role in liposome fusion and that the peptides' chirality is not necessarily required for peptide-peptide interaction within the membrane environment. Furthermore, studies along these lines may provide criteria to design protease-resistant therapeutic agents against HIV and other viruses.
Figures



References
-
- White J M. Science. 1992;258:917–924. - PubMed
-
- Veronese F D, DeVico A L, Copeland T D, Oroszlan S, Gallo R C, Sarngadharan M G. Science. 1985;229:1402–1405. - PubMed
-
- Lasky L A, Nakamura G, Smith D H, Fennie C, Shimasaki C, Patzer E, Berman P, Gregory T, Capon D J. Cell. 1987;50:975–985. - PubMed
-
- Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Science. 1987;237:1351–1355. - PubMed
-
- Gallaher W R. Cell. 1987;50:327–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

