Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection
- PMID: 9636167
- PMCID: PMC22641
- DOI: 10.1073/pnas.95.13.7433
Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection
Abstract
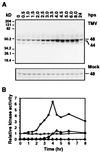
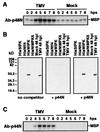
Salicylic acid-induced protein kinase (SIPK) and wounding-induced protein kinase (WIPK), two distinct members of the mitogen-activated protein (MAP) kinase family, are activated in tobacco resisting infection by tobacco mosaic virus (TMV). WIPK activation by TMV depends on the disease-resistance gene N because infection of susceptible tobacco not carrying the N gene failed to activate WIPK. Activation of WIPK required not only posttranslational phosphorylation but also a preceding rise in its mRNA and de novo synthesis of WIPK protein. The induction by TMV of WIPK mRNA and protein also occurred systemically. Its activation at the mRNA, protein, and enzyme levels was independent of salicylic acid. The regulation of WIPK at multiple levels by an N gene-mediated signal(s) suggests that this MAP kinase may be an important component upstream of salicylic acid in the signal-transduction pathway(s) leading to local and systemic resistance to TMV.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

