Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena
- PMID: 9636175
- PMCID: PMC22657
- DOI: 10.1073/pnas.95.13.7480
Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena
Abstract
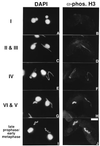
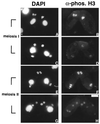
H3 phosphorylation has been correlated with mitosis temporally in mammalian cells and spatially in ciliated protozoa. In logarithmically growing Tetrahymena thermophila cells, for example, H3 phosphorylation can be detected in germline micronuclei that divide mitotically but not in somatic macronuclei that divide amitotically. Here, we demonstrate that micronuclear H3 phosphorylation occurs at a single site (Ser-10) in the amino-terminal domain of histone H3, the same site phosphorylated during mitosis in mammalian cells. Using an antibody specific for Ser-10 phosphorylated H3, we show that, in Tetrahymena, this modification is correlated with mitotic and meiotic divisions of micronuclei in a fashion that closely coincides with chromosome condensation. Our data suggest that H3 phosphorylation at Ser-10 is a highly conserved event among eukaryotes and is likely involved in both mitotic and meiotic chromosome condensation.
Figures



Similar articles
-
Phosphorylation of histone H3 is required for proper chromosome condensation and segregation.Cell. 1999 Apr 2;97(1):99-109. doi: 10.1016/s0092-8674(00)80718-7. Cell. 1999. PMID: 10199406
-
Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation.J Biol Chem. 1999 Sep 3;274(36):25543-9. doi: 10.1074/jbc.274.36.25543. J Biol Chem. 1999. PMID: 10464286
-
Phosphorylation of linker histones by cAMP-dependent protein kinase in mitotic micronuclei of Tetrahymena.Chromosoma. 1993 Nov;102(9):637-47. doi: 10.1007/BF00352312. Chromosoma. 1993. PMID: 8306826
-
Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation.Trends Genet. 2004 Apr;20(4):214-20. doi: 10.1016/j.tig.2004.02.007. Trends Genet. 2004. PMID: 15041176 Review.
-
Phosphorylation of serine 10 in histone H3, what for?J Cell Sci. 2003 Sep 15;116(Pt 18):3677-85. doi: 10.1242/jcs.00735. J Cell Sci. 2003. PMID: 12917355 Review.
Cited by
-
Abortive cell cycle events in the brains of scrapie-infected hamsters with remarkable decreases of PLK3/Cdc25C and increases of PLK1/cyclin B1.Mol Neurobiol. 2013 Dec;48(3):655-68. doi: 10.1007/s12035-013-8455-1. Epub 2013 Apr 27. Mol Neurobiol. 2013. PMID: 23625313
-
Programmed DNA Elimination in Vertebrates.Annu Rev Anim Biosci. 2021 Feb 16;9:173-201. doi: 10.1146/annurev-animal-061220-023220. Epub 2020 Sep 28. Annu Rev Anim Biosci. 2021. PMID: 32986476 Free PMC article. Review.
-
Transcriptome analysis of dormant tomonts of the marine fish ectoparasitic ciliate Cryptocaryon irritans under low temperature.Parasit Vectors. 2016 May 13;9(1):280. doi: 10.1186/s13071-016-1550-1. Parasit Vectors. 2016. PMID: 27177617 Free PMC article.
-
Nuclear CaMKII enhances histone H3 phosphorylation and remodels chromatin during cardiac hypertrophy.Nucleic Acids Res. 2013 Sep;41(16):7656-72. doi: 10.1093/nar/gkt500. Epub 2013 Jun 26. Nucleic Acids Res. 2013. Retraction in: Nucleic Acids Res. 2023 Mar 21;51(5):2500. doi: 10.1093/nar/gkad112. PMID: 23804765 Free PMC article. Retracted.
-
SET8-mediated methylations of histone H4 lysine 20 mark silent heterochromatic domains in apicomplexan genomes.Mol Cell Biol. 2007 Aug;27(16):5711-24. doi: 10.1128/MCB.00482-07. Epub 2007 Jun 11. Mol Cell Biol. 2007. PMID: 17562855 Free PMC article.
References
-
- Luger K, Mader A W, Richmond R K, Sargent D F, Richmond T J. Nature (London) 1997;389:251–260. - PubMed
-
- Wolffe A P. Chromatin: Structure and Function. San Diego: Academic; 1995. pp. 72–77.
-
- Bradbury E M. BioEssays. 1992;14:9–16. - PubMed
-
- Roth S Y, Allis C D. Trends Biochem Sci. 1992;17:93–98. - PubMed
-
- Shen X, Yu L, Weir J W, Gorovsky M A. Cell. 1995;82:47–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

