Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope
- PMID: 9636186
- PMCID: PMC22678
- DOI: 10.1073/pnas.95.13.7544
Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope
Abstract
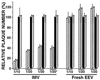
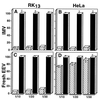
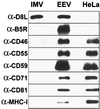
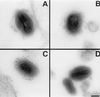
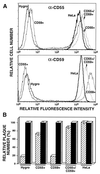
Vaccinia virus (VV) produces two antigenically and structurally distinct infectious virions, intracellular mature virus (IMV) and extracellular enveloped virus (EEV). Here we have investigated the resistance of EEV and IMV to neutralization by complement in the absence of immune antibodies. When EEV is challenged with complement from the same species as the cells used to grow the virus, EEV is resistant to neutralization by complement, whereas IMV is not. EEV resistance was not a result of EEV protein B5R, despite its similarity to proteins of the regulators of complement activation (RCA) family, or to any of the other EEV proteins tested (A34R, A36R, and A56R gene products). EEV was sensitive to complement when the virus was grown in one species and challenged with complement from a different species, suggesting that complement resistance might be mediated by host RCA incorporated into the EEV outer envelope. This hypothesis was confirmed by several observations: (i) immunoblot analysis revealed that cellular membrane proteins CD46, CD55, CD59, CD71, CD81, and major histocompatibility complex class I antigen were detected in purified EEV but not IMV; (ii) immunoelectron microscopy revealed cellular RCA on the surface of EEV retained on the cell surface; and (iii) EEV derived from rat cells expressing the human RCA CD55 or CD55 and CD59 were more resistant to human complement than EEV derived from control rat cells that expressed neither CD55 nor CD59. These data justify further analysis of the roles of these (and possible other) cellular proteins in EEV biology.
Figures
References
-
- Sim R B, Dodds A W. In: Complement. A Practical Approach. Dodds A W, Sim R B, editors. Oxford: IRL; 1997. pp. 1–18.
-
- Law S K A, Reid K B M. Complement. Oxford: IRL; 1995.
-
- Cooper N R. Immunol Today. 1991;12:327–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

