Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice
- PMID: 9636188
- PMCID: PMC22681
- DOI: 10.1073/pnas.95.13.7556
Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice
Abstract
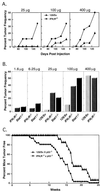
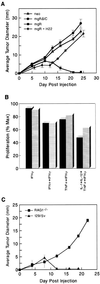
This study demonstrates that endogenously produced interferon gamma (IFN-gamma) forms the basis of a tumor surveillance system that controls development of both chemically induced and spontaneously arising tumors in mice. Compared with wild-type mice, mice lacking sensitivity to either IFN-gamma (i.e., IFN-gamma receptor-deficient mice) or all IFN family members (i.e., Stat1-deficient mice) developed tumors more rapidly and with greater frequency when challenged with different doses of the chemical carcinogen methylcholanthrene. In addition, IFN-gamma-insensitive mice developed tumors more rapidly than wild-type mice when bred onto a background deficient in the p53 tumor-suppressor gene. IFN-gamma-insensitive p53(-/-) mice also developed a broader spectrum of tumors compared with mice lacking p53 alone. Using tumor cells derived from methylcholanthrene-treated IFN-gamma-insensitive mice, we found IFN-gamma's actions to be mediated at least partly through its direct effects on the tumor cell leading to enhanced tumor cell immunogenicity. The importance and generality of this system is evidenced by the finding that certain types of human tumors become selectively unresponsive to IFN-gamma. Thus, IFN-gamma forms the basis of an extrinsic tumor-suppressor mechanism in immunocompetent hosts.
Figures


References
-
- Farrar M A, Schreiber R D. Annu Rev Immunol. 1993;11:571–611. - PubMed
-
- Boehm U, Klamp T, Groot M, Howard J C. Annu Rev Immunol. 1997;15:749–795. - PubMed
-
- Bach E A, Aguet M, Schreiber R D. Annu Rev Immunol. 1997;15:563–591. - PubMed
-
- Darnell J E, Jr, Kerr I M, Stark G R. Science. 1994;264:1415–1421. - PubMed
-
- Meraz M A, White J M, Sheehan K C F, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, et al. Cell. 1996;84:431–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

