Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)
- PMID: 9636206
- PMCID: PMC22714
- DOI: 10.1073/pnas.95.13.7659
Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)
Abstract
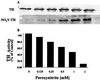
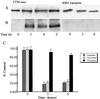
The decrement in dopamine levels exceeds the loss of dopaminergic neurons in Parkinson's disease (PD) patients and experimental models of PD. This discrepancy is poorly understood and may represent an important event in the pathogenesis of PD. Herein, we report that the rate-limiting enzyme in dopamine synthesis, tyrosine hydroxylase (TH), is a selective target for nitration following exposure of PC12 cells to either peroxynitrite or 1-methyl-4-phenylpyridiniun ion (MPP+). Nitration of TH also occurs in mouse striatum after MPTP administration. Nitration of tyrosine residues in TH results in loss of enzymatic activity. In the mouse striatum, tyrosine nitration-mediated loss in TH activity parallels the decline in dopamine levels whereas the levels of TH protein remain unchanged for the first 6 hr post MPTP injection. Striatal TH was not nitrated in mice overexpressing copper/zinc superoxide dismutase after MPTP administration, supporting a critical role for superoxide in TH tyrosine nitration. These results indicate that tyrosine nitration-induced TH inactivation and consequently dopamine synthesis failure, represents an early and thus far unidentified biochemical event in MPTP neurotoxic process. The resemblance of the MPTP model with PD suggests that a similar phenomenon may occur in PD, influencing the severity of parkisonian symptoms.
Figures


References
-
- Fahn S. In: Cecil’s Textbook of Medicine. Wyngaarden J B, Smith LH Jr, editors. Philadelphia: Saunders; 1988. pp. 2143–2147.
-
- Hornykiewicz O, Kish S J. In: Parkinson’s Disease. Yahr M, Bergmann K J, editors. New York: Raven; 1987. pp. 19–34.
-
- Jackson-Lewis V, Jakowec M, Burke R E, Przedborski S. Neurodegener. 1995;4:257–269. - PubMed
-
- Seniuk N A, Tatton W G, Greenwood C E. Brain Res. 1990;527:7–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

