A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii
- PMID: 9636211
- PMCID: PMC22724
- DOI: 10.1073/pnas.95.13.7687
A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii
Abstract
Classical quorum-sensing (autoinduction) regulation, as exemplified by the lux system of Vibrio fischeri, requires N-acyl homoserine lactone (AHL) signals to stimulate cognate transcriptional activators for the cell density-dependent expression of specific target gene systems. For Pantoea stewartii subsp. stewartii, a bacterial pathogen of sweet corn and maize, the extracellular polysaccharide (EPS) stewartan is a major virulence factor, and its production is controlled by quorum sensing in a population density-dependent manner. Two genes, esaI and esaR, encode essential regulatory proteins for quorum sensing. EsaI is the AHL signal synthase, and EsaR is the cognate gene regulator. esaI, DeltaesaR, and DeltaesaI-esaR mutations were constructed to establish the regulatory role of EsaR. We report here that strains containing an esaR mutation produce high levels of EPS independently of cell density and in the absence of the AHL signal. Our data indicate that quorum-sensing regulation in P. s. subsp. stewartii, in contrast to most other described systems, uses EsaR to repress EPS synthesis at low cell density, and that derepression requires micromolar amounts of AHL. In addition, derepressed esaR strains, which synthesize EPS constitutively at low cell densities, were significantly less virulent than the wild-type parent. This finding suggests that quorum sensing in P. s. subsp. stewartii may be a mechanism to delay the expression of EPS during the early stages of infection so that it does not interfere with other mechanisms of pathogenesis.
Figures


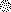 ...)
were analyzed for the presence of bound and free EPS. The data points
indicated for each strain are the combined data obtained from three
separate experiments. The amount of EPS (y axis) is
expressed as pg/cell. The number of colony-forming units (CFU),
expressed as Log10 cfu/ml, was determined by dilution
plating on nutrient agar. (B) Qualitative analysis of EPS
production on nutrient agar medium (Left) and CPG medium
(Right). The wild-type strain DC283 exhibits a mucoid
phenotype only on CPG medium, whereas the ΔesaR and
ΔesaIR mutants, ESΔR and ESΔIR, are mucoid on both
media. The mutant strain ESN51 is unable to synthesize EPS under either
condition.
...)
were analyzed for the presence of bound and free EPS. The data points
indicated for each strain are the combined data obtained from three
separate experiments. The amount of EPS (y axis) is
expressed as pg/cell. The number of colony-forming units (CFU),
expressed as Log10 cfu/ml, was determined by dilution
plating on nutrient agar. (B) Qualitative analysis of EPS
production on nutrient agar medium (Left) and CPG medium
(Right). The wild-type strain DC283 exhibits a mucoid
phenotype only on CPG medium, whereas the ΔesaR and
ΔesaIR mutants, ESΔR and ESΔIR, are mucoid on both
media. The mutant strain ESN51 is unable to synthesize EPS under either
condition.


References
-
- Fuqua W C, Winans S C, Greenberg E P. Annu Rev Microbiol. 1996;50:727–751. - PubMed
-
- Sitnikov D M, Schineller J B, Baldwin T O. Mol Microbiol. 1995;17:801–812. - PubMed
-
- Swift S, Throup J P, Williams P, Salmond G P C, Stewart G S A B. Trends Biochem Sci. 1996;21:214–219. - PubMed
-
- Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Biochemistry. 1981;20:2444–2449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

