Cyclic AMP regulates potassium channel expression in C6 glioma by destabilizing Kv1.1 mRNA
- PMID: 9636212
- PMCID: PMC22725
- DOI: 10.1073/pnas.95.13.7693
Cyclic AMP regulates potassium channel expression in C6 glioma by destabilizing Kv1.1 mRNA
Abstract
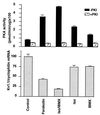
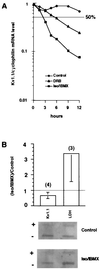
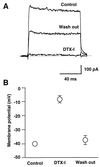
The tissue distributions and physiological properties of a variety of cloned voltage-gated potassium channel genes have been characterized extensively, yet relatively little is known about the mechanisms controlling expression of these genes. Here, we report studies on the regulation of Kv1.1 expressed endogenously in the C6 glioma cell line. We demonstrate that elevation of intracellular cAMP leads to the accelerated degradation of Kv1.1 RNA. The cAMP-induced decrease in Kv1.1 RNA is followed by a decrease in Kv1. 1 protein and a decrease in the whole cell sustained K+ current amplitude. Dendrotoxin-I, a relatively specific blocker of Kv1.1, blocks 96% of the sustained K+ current in glioma cells, causing a shift in the resting membrane potential from -40 mV to -7 mV. These data suggest that expression of Kv1.1 contributes to setting the resting membrane potential in undifferentiated glioma cells. We therefore suggest that receptor-mediated elevation of cAMP reduces outward K+ current density by acting at the translational level to destabilize Kv1.1 RNA, an additional mechanism for regulating potassium channel gene expression.
Figures
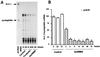

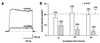
References
-
- Mori Y, Matsubara H, Folco E, Siegel A, Koren G. J Biol Chem. 1993;268:26482–26493. - PubMed
-
- Mori Y, Folco E, Koren G. J Biol Chem. 1995;270:27788–27796. - PubMed
-
- Takimoto K, Levitan E S. Biochemistry. 1996;35:14149–14156. - PubMed
-
- Levitan E S, Gealy R, Trimmer J S, Takimoto K. J Biol Chem. 1995;270:6036–60341. - PubMed
-
- Gan L, Perney T M, Kaczmarek L K. J Biol Chem. 1996;271:5859–5865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

