Conformational changes couple Na+ and glucose transport
- PMID: 9636229
- PMCID: PMC22758
- DOI: 10.1073/pnas.95.13.7789
Conformational changes couple Na+ and glucose transport
Abstract
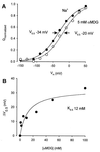
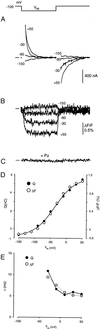
The mechanism by which cotransport proteins couple their substrates across cell membranes is not known. A commonly proposed model is that cotransport results from ligand-induced conformational transitions that change the accessibility of ligand-binding sites from one side of the membrane to the other. To test this model, we have measured the accessibility of covalent probes to a cysteine residue (Q457C) placed in the putative sugar-translocation domain of the Na+/glucose cotransporter (SGLT1). The mutant protein Q457C was able to transport sugar, but transport was abolished after alkylation by methanethiosulfonate reagents. Alkylation blocked sugar translocation but not sugar binding. Accessibility of Q457C to alkylating reagents required external Na+ and was blocked by external sugar and phlorizin. The voltage dependence of accessibility was directly correlated with the presteady-state charge movement of SGLT1. Voltage-jump experiments with rhodamine-6-maleimide-labeled Q457C showed that the time course and level of changes in fluorescence closely followed the presteady-state charge movement. We conclude that conformational changes are responsible for the coupling of Na+ and sugar transport and that Q457 plays a critical role in sugar translocation by SGLT1.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

