Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis
- PMID: 9636235
- PMCID: PMC22771
- DOI: 10.1073/pnas.95.13.7825
Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis
Abstract
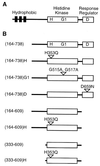
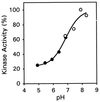
ETR1 represents a prototypical ethylene receptor. Homologues of ETR1 have been identified in Arabidopsis as well as in other plant species, indicating that ethylene perception involves a family of receptors and that the mechanism of ethylene perception is conserved in plants. The amino-terminal half of ETR1 contains a hydrophobic domain responsible for ethylene binding and membrane localization. The carboxyl-terminal half of the polypeptide contains domains with homology to histidine kinases and response regulators, signaling motifs originally identified in bacteria. The putative histidine kinase domain of ETR1 was expressed in yeast as a fusion protein with glutathione S-transferase and affinity purified. Autophosphorylation of the purified fusion protein was observed on incubation with radiolabeled ATP. The incorporated phosphate was resistant to treatment with 3 M NaOH, but was sensitive to 1 M HCl, consistent with phosphorylation of histidine. Autophosphorylation was abolished by mutations that eliminated either the presumptive site of phosphorylation (His-353) or putative catalytic residues within the kinase domain. Truncations were used to delineate the region required for histidine kinase activity. An examination of cation requirements indicated that ETR1 requires Mn2+ for autophosphorylation. These results demonstrate that higher plants contain proteins with histidine kinase activity. Furthermore, these results indicate that aspects of ethylene signaling may be regulated by changes in histidine kinase activity of the receptor.
Figures



Similar articles
-
Mutational analysis of the ethylene receptor ETR1. Role of the histidine kinase domain in dominant ethylene insensitivity.Plant Physiol. 2002 Apr;128(4):1428-38. doi: 10.1104/pp.010777. Plant Physiol. 2002. PMID: 11950991 Free PMC article.
-
Ethylene controls autophosphorylation of the histidine kinase domain in ethylene receptor ETR1.Mol Plant. 2008 Mar;1(2):380-7. doi: 10.1093/mp/ssn004. Epub 2008 Feb 11. Mol Plant. 2008. PMID: 19825547
-
Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission.Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):352-7. doi: 10.1073/pnas.0237085100. Epub 2002 Dec 30. Proc Natl Acad Sci U S A. 2003. PMID: 12509505 Free PMC article.
-
The ethylene-receptor family from Arabidopsis: structure and function.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1405-12. doi: 10.1098/rstb.1998.0295. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800203 Free PMC article. Review.
-
Ethylene and cytokinin signalling in plants: the role of two-component systems.Essays Biochem. 1997;32:101-11. Essays Biochem. 1997. PMID: 9493014 Review.
Cited by
-
Arabidopsis ETR1 and ERS1 differentially repress the ethylene response in combination with other ethylene receptor genes.Plant Physiol. 2012 Mar;158(3):1193-207. doi: 10.1104/pp.111.187757. Epub 2012 Jan 6. Plant Physiol. 2012. PMID: 22227969 Free PMC article.
-
Ethylene receptors in plants - why so much complexity?F1000Prime Rep. 2015 Apr 2;7:39. doi: 10.12703/P7-39. eCollection 2015. F1000Prime Rep. 2015. PMID: 26171216 Free PMC article. Review.
-
The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor.Plant Physiol. 1999 Sep;121(1):291-300. doi: 10.1104/pp.121.1.291. Plant Physiol. 1999. PMID: 10482685 Free PMC article.
-
Evidence of the functional role of the ethylene receptor genes SlETR4 and SlETR5 in ethylene signal transduction in tomato.Mol Genet Genomics. 2019 Apr;294(2):301-313. doi: 10.1007/s00438-018-1505-7. Epub 2018 Oct 31. Mol Genet Genomics. 2019. PMID: 30382349
-
Ethylene signaling in plants.J Biol Chem. 2020 May 29;295(22):7710-7725. doi: 10.1074/jbc.REV120.010854. Epub 2020 Apr 24. J Biol Chem. 2020. PMID: 32332098 Free PMC article. Review.
References
-
- Parkinson J S. Cell. 1993;73:857–871. - PubMed
-
- Swanson R V, Alex L A, Simon M I. Trends Biochem. 1994;19:485–490. - PubMed
-
- Stock J B, Surette M G, Levit M, Park P. In: Two-Component Signal Transduction. Hoch J A, Silhavy T J, editors. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 25–51.
-
- Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Cell. 1996;86:865–875. - PubMed
-
- Popov K M, Zhao Y, Shimomura Y, Kuntz M J, Harris R A. J Biol Chem. 1992;267:13127–13130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

