Seven-up, the Drosophila homolog of the COUP-TF orphan receptors, controls cell proliferation in the insect kidney
- PMID: 9637680
- PMCID: PMC316909
- DOI: 10.1101/gad.12.12.1781
Seven-up, the Drosophila homolog of the COUP-TF orphan receptors, controls cell proliferation in the insect kidney
Abstract
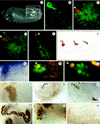
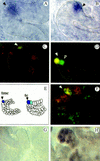
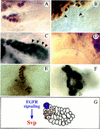
Morphogenesis of the insect kidney, the Malpighian tubules, is controlled in Drosophila by a single large cell, the tip cell. It has been postulated that this cell sends out a mitogenic signal that induces the division of neighboring cells. The signal and the molecules that receive it have remained elusive. We show that the COUP-TF-related nuclear orphan receptor Seven-up is a key component that becomes induced in response to mitogenic EGF receptor signaling activity emanating from the tip cell. Seven-up in turn is capable of regulating the transcription of cell cycle regulators.
Figures

Similar articles
-
A genetic hierarchy establishes mitogenic signalling and mitotic competence in the renal tubules of Drosophila.Development. 2002 Feb;129(4):935-44. doi: 10.1242/dev.129.4.935. Development. 2002. PMID: 11861476
-
Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization.EMBO J. 1997 Apr 1;16(7):1656-69. doi: 10.1093/emboj/16.7.1656. EMBO J. 1997. PMID: 9130711 Free PMC article.
-
Stat 5b and the orphan nuclear receptors regulate expression of the alpha2-macroglobulin (alpha2M) gene in rat ovarian granulosa cells.Mol Endocrinol. 1998 Sep;12(9):1393-409. doi: 10.1210/mend.12.9.0161. Mol Endocrinol. 1998. PMID: 9731707
-
Physiological function of the orphans GCNF and COUP-TF.Trends Endocrinol Metab. 2001 Aug;12(6):247-51. doi: 10.1016/s1043-2760(01)00424-6. Trends Endocrinol Metab. 2001. PMID: 11445441 Review.
-
[Gene cascade by hormonal control during development of insects].Tanpakushitsu Kakusan Koso. 2003 Dec;48(16):2254-60. Tanpakushitsu Kakusan Koso. 2003. PMID: 14661583 Review. Japanese. No abstract available.
Cited by
-
Female-specific wing degeneration caused by ecdysteroid in the Tussock Moth, Orgyia recens: hormonal and developmental regulation of sexual dimorphism.J Insect Sci. 2003;3:11. doi: 10.1093/jis/3.1.11. Epub 2003 Apr 29. J Insect Sci. 2003. PMID: 15841227 Free PMC article.
-
Sites of Fgf signalling and perception during embryogenesis of the beetle Tribolium castaneum.Dev Genes Evol. 2008 Apr;218(3-4):153-67. doi: 10.1007/s00427-007-0192-x. Epub 2008 Apr 8. Dev Genes Evol. 2008. PMID: 18392877
-
Identification of genes controlling malpighian tubule and other epithelial morphogenesis in Drosophila melanogaster.Genetics. 1999 Feb;151(2):685-95. doi: 10.1093/genetics/151.2.685. Genetics. 1999. PMID: 9927461 Free PMC article.
-
Release and spread of Wingless is required to pattern the proximo-distal axis of Drosophila renal tubules.Elife. 2018 Aug 10;7:e35373. doi: 10.7554/eLife.35373. Elife. 2018. PMID: 30095068 Free PMC article.
-
The function of nuclear receptors in regulation of female reproduction and embryogenesis in the red flour beetle, Tribolium castaneum.J Insect Physiol. 2010 Oct;56(10):1471-80. doi: 10.1016/j.jinsphys.2010.04.004. Epub 2010 Apr 27. J Insect Physiol. 2010. PMID: 20416316 Free PMC article.
References
-
- Baumann, P. and H. Skaer. 1993. The Drosophila EGFR homolog (DER) is required for Malpighian tubule development. Development (Suppl.) 65–75. - PubMed
-
- Begemann G, Michon A-M, v.d. Voorn L, Wepf R, Mlodzik M. The Drosophila orphan nuclear receptor Seven-up requires the Ras pathway for its function in photoreceptor determination. Development. 1995;121:225–237. - PubMed
-
- Bier E, Jan LY, Jan YN. rhomboid, a gene required for dorsoventral Drosophila melanogaster. Genes & Dev. 1990;4:190–203. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. New York, NY: Springer Verlag; 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
