The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation
- PMID: 9637688
- PMCID: PMC316912
- DOI: 10.1101/gad.12.12.1871
The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation
Abstract
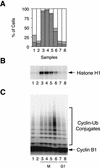
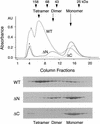
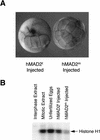
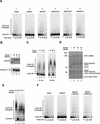
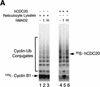
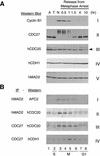
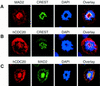
The spindle assembly checkpoint mechanism delays anaphase initiation until all chromosomes are aligned at the metaphase plate. Activation of the anaphase-promoting complex (APC) by binding of CDC20 and CDH1 is required for exit from mitosis, and APC has been implicated as a target for the checkpoint intervention. We show that the human checkpoint protein hMAD2 prevents activation of APC by forming a hMAD2-CDC20-APC complex. When injected into Xenopus embryos, hMAD2 arrests cells at mitosis with an inactive APC. The recombinant hMAD2 protein exists in two-folded states: a tetramer and a monomer. Both the tetramer and the monomer bind to CDC20, but only the tetramer inhibits activation of APC and blocks cell cycle progression. Thus, hMAD2 binding is not sufficient for inhibition, and a change in hMAD2 structure may play a role in transducing the checkpoint signal. There are at least three different forms of mitotic APC that can be detected in vivo: an inactive hMAD2-CDC20-APC ternary complex present at metaphase, a CDC20-APC binary complex active in degrading specific substrates at anaphase, and a CDH1-APC complex active later in mitosis and in G1. We conclude that the checkpoint-mediated cell cycle arrest involves hMAD2 receiving an upstream signal to inhibit activation of APC.
Figures


References
-
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. - PubMed
-
- Chen RH, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. - PubMed
-
- Clute P, Masui Y. Regulation of the appearance of division asynchrony and microtubule-dependent chromosome cycles in Xenopus laevis embryos. Dev Biol. 1995;171:273–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
