1,25-Dihydroxyvitamin D3 restores sensitivity to cyclophosphamide-induced apoptosis in non-obese diabetic (NOD) mice and protects against diabetes
- PMID: 9649179
- PMCID: PMC1904978
- DOI: 10.1046/j.1365-2249.1998.00568.x
1,25-Dihydroxyvitamin D3 restores sensitivity to cyclophosphamide-induced apoptosis in non-obese diabetic (NOD) mice and protects against diabetes
Abstract
The activated form of vitamin D, 1,25(OH)2D3, and its analogues can prevent type I diabetes in NOD mice. Protection is achieved without signs of systemic immunosuppression and is associated with a restoration of the defective immune regulator system of the NOD mice. The aim of the present study was to investigate whether this restoration of regulator cell function is the only mechanism in the prevention of diabetes by 1,25(OH)2D3. We tested therefore if 1,25(OH)2D3 could prevent cyclophosphamide-induced diabetes, since diabetes occurring after cyclophosphamide injection is believed to be due to an elimination of suppresser cells. NOD mice treated with 1,25(OH)2D3 (5 microg/kg every 2 days) from the time of weaning were clearly protected against diabetes induced by cyclophosphamide (200 mg/kg body wt at 70 days old) (2/12 (17%) versus 36/53 (68%) in control mice, P < 0.005). By co-transfer experiments it was demonstrated that cyclophosphamide had indeed eliminated the suppresser cells present in 1,25(OH)2D3-treated mice. Since cyclophosphamide injection did not break the protection offered by 1,25(OH)2D3, it was clear that diabetogenic effector cells were affected by 1,25(OH)2D3 treatment as well. This was confirmed by the finding that splenocytes from 1,25(OH)2D3-treated mice were less capable of transferring diabetes in young, irradiated NOD mice, and by the demonstration of lower Th1 cytokine levels in the pancreases of 1,25(OH)2D3-treated, cyclophosphamide-injected mice. This better elimination of effector cells in 1,25(OH)2D3-treated mice could be explained by a restoration of the sensitivity to cyclophosphamide-induced apoptosis in both thymocytes and splenocytes, in normally apoptosis-resistant NOD mice. Altogether, these data indicate that the protection against diabetes offered by 1,25(OH)2D3 may be independent of the presence of suppresser cells, and may involve increased apoptosis of Th1 autoimmune effector cells.
Figures

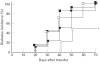

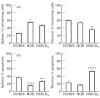

References
-
- Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes. 1992;41:1491–5. - PubMed
-
- Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of type I diabetes in NOD mice by 1,25-dihydroxyvitamin D3. Diabetologia. 1994;37:552–8. - PubMed
-
- Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia. 1984;27:604–6. - PubMed
-
- Yasunami R, Bach JF. Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol. 1988;18:481–4. - PubMed
-
- Mitsuoka A, Baba M, Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976;262:77–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

