Isolation and phenotypic characterization of colonic macrophages
- PMID: 9649182
- PMCID: PMC1904962
- DOI: 10.1046/j.1365-2249.1998.00557.x
Isolation and phenotypic characterization of colonic macrophages
Abstract
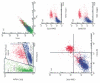
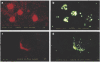
Macrophages play an important role in the intestinal mucosal immune system. However, they are a poorly defined cell population. We therefore determined their phenotype in normal colonic mucosa. Macrophages were isolated from colonic biopsies and surgical specimens by collagenase digestion. Colonic macrophages were positively sorted by anti-CD33 magnetic beads. Flow cytometric triple fluorescence analysis was applied to study CD14, CD16, CD33, CD44, CD11b, CD11c, CD64, HLA-DR, CD80, CD86 and CD3/CD19 expression. CD33 was evaluated as a positive marker for intestinal macrophages. CD33+ cells isolated from normal colonic mucosa showed co-expression of the established intracellular macrophage marker CD68 in FACS analysis. CD33+ cells were capable of phagocytosis. Isolation of this cell population by magnetic anti-CD33 beads and culture resulted in a 4.2-40-fold increase in IL-1beta and 4.5-44-fold increase in tumour necrosis factor-alpha (TNF-alpha) secretion compared with unsorted lamina propria mononuclear cells (LPMC). Of the CD33+ cells, 90.9 +/- 6.9% (mean +/- s.d.) were CD44+. However, macrophages from colonic mucosa showed only a low expression of CD14 (10.5 +/- 3.8%), CD16 (10.1 +/- 3.9%), HLA-DR (27.3 +/- 9.2%), CD11b (17.4 +/- 6.8%), CD11c (17.8 +/- 10.4%). Furthermore, expression of CD80 (9.2 +/- 4.2%) and CD86 (15.1 +/- 7.3%) was low, suggesting a low ability of normal intestinal macrophages to activate T cells and T cell-mediated immune responses. We conclude that CD33 is useful for the isolation and flow cytometric characterization of colonic macrophages. These cells exhibit a single phenotype in normal mucosa (CD33++, CD44++, CD14-, CD16-, CD11b-, CD11c-, HLA-DRlow, CD80-, CD86-) lacking lipopolysaccharide (LPS) receptor and costimulatory molecules.
Figures







References
-
- Pavli P, Doe WF. Intestinal macrophages. In: MacDermott RP, Stenson WF, editors. Inflammatory bowel disease. New York: Elsevier; 1992. pp. 177–88.
-
- Donnellan WL. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterol. 1965;49:496–514. - PubMed
-
- Andus T, Rogler G, Daig R, Falk W, Schölmerich J, Gross V. Inflammatory bowel disease. In: Tygat GNJ, Bartelsman JFWM, van Deventer SJH, editors. The role of macrophages. Dordrecht: Kluwer: 1995. pp. 281–97.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

