Functional intertwining of Dpp and EGFR signaling during Drosophila endoderm induction
- PMID: 9649506
- PMCID: PMC316971
- DOI: 10.1101/gad.12.13.2022
Functional intertwining of Dpp and EGFR signaling during Drosophila endoderm induction
Abstract
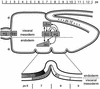
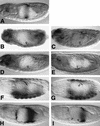
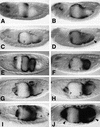
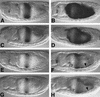
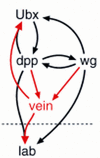
Endoderm induction in Drosophila is mediated by the extracellular signals Decapentaplegic (Dpp) and Wingless (Wg). We discovered a secondary signal with a permissive role in this process, namely Vein, a neuregulin-like ligand that stimulates the epidermal growth factor receptor (EGFR) and Ras signaling. Dpp and Wg up-regulate vein expression in the midgut mesoderm in two regions overlapping the Dpp sources. Experiments based on lack of function and ectopic stimulation of Dpp and EGFR signaling show that these two pathways are functionally interdependent and that they synergize with each other, revealing functional intertwining. The transcriptional response elements for the Dpp signal in midgut enhancers from homeotic target genes are bipartite, comprising CRE sites as well as binding sites for the Dpp signal-transducing protein Mad. Of these sites, the CRE seems to function primarily in the response to Ras, the secondary signal of Dpp. We discuss the potential significance of why an inductive process might use a secondary signal whose function is intertwined with that of the primary signal.
Figures



References
-
- Arora K, Dai H, Kazuko SG, Jamal J, O’Connor MB, Letsou A, Warrior R. The Drosophila schnurri gene acts in the Dpp/TGF-β signaling pathway and encodes a transcription factor homologous to the human MBP family. Cell. 1995;81:781–790. - PubMed
-
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by Hedgehog protein. Nature. 1994;368:208–214. - PubMed
-
- Bienz M. Endoderm induction in Drosophila: The nuclear targets of the inducing signals. Curr Opin Genet Dev. 1997;7:683–688. - PubMed
-
- Bier E, Jan LY, Jan YN. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes & Dev. 1990;4:190–203. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
