The voltage dependence of a cloned mammalian renal type II Na+/Pi cotransporter (NaPi-2)
- PMID: 9649580
- PMCID: PMC2229411
- DOI: 10.1085/jgp.112.1.1
The voltage dependence of a cloned mammalian renal type II Na+/Pi cotransporter (NaPi-2)
Abstract
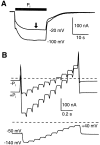
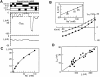
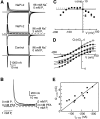
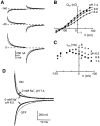
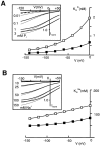
The voltage dependence of the rat renal type II Na+/Pi cotransporter (NaPi-2) was investigated by expressing NaPi-2 in Xenopus laevis oocytes and applying the two-electrode voltage clamp. In the steady state, superfusion with inorganic phosphate (Pi) induced inward currents (Ip) in the presence of 96 mM Na+ over the potential range -140 </= V </= +40 mV. With Pi as the variable substrate, the apparent affinity constant (KmPi) was strongly dependent on Na+, increasing sixfold for a twofold reduction in external Na+. KmPi increased with depolarizing voltage and was more sensitive to voltage at reduced Na+. The Hill coefficient was close to unity and the predicted maximum Ip (Ipmax) was 40% smaller at 50 mM Na+. With Na+ as the variable substrate, KmNa was weakly dependent on both Pi and voltage, the Hill coefficient was close to 3 and Ipmax was independent of Pi at -50 mV. The competitive inhibitor phosphonoformic acid suppressed the steady state holding current in a Na+-dependent manner, indicating the existence of uncoupled Na+ slippage. Voltage steps induced pre-steady state relaxations typical for Na+-coupled cotransporters. NaPi-2-dependent relaxations were quantitated by a single, voltage-dependent exponential. At 96 mM Na+, a Boltzmann function was fit to the steady state charge distribution (Q-V) to give a midpoint voltage (V0.5) in the range -20 to -50 mV and an apparent valency of approximately 0.5 e-. V0.5 became more negative as Na+ was reduced. Pi suppressed relaxations in a dose-dependent manner, but had little effect on their voltage dependence. Reducing external pH shifted V0.5 to depolarizing potentials and suppressed relaxations in the absence of Na+, suggesting that protons interact with the unloaded carrier. These findings were incorporated into an ordered kinetic model whereby Na+ is the first and last substrate to bind, and the observed voltage dependence arises from the unloaded carrier and first Na+ binding step.
Figures





References
-
- Adrian RH. Charge movement in the membrane of striated muscle. Annu Rev Biophys Bioeng. 1978;7:85–112. - PubMed
-
- Amstutz M, Mohrmann M, Gmaj P, Murer H. Effect of pH on phosphate transport in rat renal brush border vesicles. Am J Physiol. 1985;248:F705–F710. - PubMed
-
- Bennett E, Kimmich GS. Na+ binding to the Na+-glucose cotransporter is potential dependent. Am J Physiol. 1992;262:C510–C516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

