Macroscopic Na+ currents in the "Nonconducting" Shaker potassium channel mutant W434F
- PMID: 9649585
- PMCID: PMC2229408
- DOI: 10.1085/jgp.112.1.85
Macroscopic Na+ currents in the "Nonconducting" Shaker potassium channel mutant W434F
Abstract
C-type inactivation in Shaker potassium channels inhibits K+ permeation. The associated structural changes appear to involve the outer region of the pore. Recently, we have shown that C-type inactivation involves a change in the selectivity of the Shaker channel, such that C-type inactivated channels show maintained voltage-sensitive activation and deactivation of Na+ and Li+ currents in K+-free solutions, although they show no measurable ionic currents in physiological solutions. In addition, it appears that the effective block of ion conduction produced by the mutation W434F in the pore region may be associated with permanent C-type inactivation of W434F channels. These conclusions predict that permanently C-type inactivated W434F channels would also show Na+ and Li+ currents (in K+-free solutions) with kinetics similar to those seen in C-type-inactivated Shaker channels. This paper confirms that prediction and demonstrates that activation and deactivation parameters for this mutant can be obtained from macroscopic ionic current measurements. We also show that the prolonged Na+ tail currents typical of C-type inactivated channels involve an equivalent prolongation of the return of gating charge, thus demonstrating that the kinetics of gating charge return in W434F channels can be markedly altered by changes in ionic conditions.
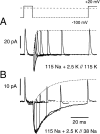
Figures




References
-
- Baukrowitz T, Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+channel. Science. 1996;271:653–656. - PubMed
-
- Bezanilla F, Stefani E. Voltage-dependent gating of ionic channels. Annu Rev Biophys Biomol Struct. 1994;23:819–846. - PubMed
-
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. - PubMed
-
- Heinemann SH, Conti F, Stühmer W. Recording of gating currents from Xenopusoocytes and gating noise analysis. Methods Enzymol. 1992;207:353–368. - PubMed

