Monoclonal antibody against Babesia equi: characterization and potential application of antigen for serodiagnosis
- PMID: 9650921
- PMCID: PMC104937
- DOI: 10.1128/JCM.36.7.1835-1839.1998
Monoclonal antibody against Babesia equi: characterization and potential application of antigen for serodiagnosis
Abstract
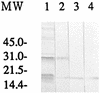
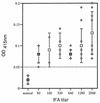
Monoclonal antibody (MAb) BEG3 was produced against Babesia equi parasites to define a species-specific antigen for diagnostic use. The MAb reacted with single, paired, and Maltese cross forms of B. equi, and no reaction was observed with this MAb on acetone-fixed Babesia caballi, Babesia ovata, or Babesia microti parasites in the indirect immunofluorescent antibody test. Confocal laser and immunoelectron microscopic studies showed that the antigen which was recognized by this MAb was located on the surface of B. equi parasites. This MAb recognized a 19-kDa protein of B. equi antigen and did not react with B. caballi antigen or normal horse erythrocytes in immunoblot analysis. This MAb also significantly inhibited the in vitro growth of the B. equi parasite. Preliminary studies using partially purified antigen, which was separated by high-pressure liquid chromatography and recognized by the MAb, suggested that it is a suitable antigen for enzyme-linked immunosorbent assay detection of anti-B. equi antibodies in naturally infected horse sera.
Figures





Similar articles
-
Monoclonal antibodies against Babesia caballi and Babesia equi and their application in serodiagnosis.Vet Parasitol. 1997 Jan;68(1-2):11-26. doi: 10.1016/s0304-4017(96)01074-6. Vet Parasitol. 1997. PMID: 9066047
-
Inhibitory effect of monoclonal antibodies on the growth of Babesia caballi.Int J Parasitol. 1999 Nov;29(11):1785-91. doi: 10.1016/s0020-7519(99)00137-x. Int J Parasitol. 1999. PMID: 10616924
-
Cloning of a novel Babesia equi gene encoding a 158-kilodalton protein useful for serological diagnosis.Clin Diagn Lab Immunol. 2005 Feb;12(2):334-8. doi: 10.1128/CDLI.12.2.334-338.2005. Clin Diagn Lab Immunol. 2005. PMID: 15699430 Free PMC article.
-
Immunochromatographic test for simultaneous serodiagnosis of Babesia caballi and B. equi infections in horses.Clin Vaccine Immunol. 2006 May;13(5):553-5. doi: 10.1128/CVI.13.5.553-555.2006. Clin Vaccine Immunol. 2006. PMID: 16682475 Free PMC article.
-
Recently developed methods for the detection of babesial infections.Trans R Soc Trop Med Hyg. 1989;83 Suppl:21-3. doi: 10.1016/0035-9203(89)90598-1. Trans R Soc Trop Med Hyg. 1989. PMID: 2696156 Review.
Cited by
-
Detection of antibodies to Babesia equi in horses by a latex agglutination test using recombinant EMA-1.Clin Diagn Lab Immunol. 2001 May;8(3):645-6. doi: 10.1128/CDLI.8.3.645-646.2001. Clin Diagn Lab Immunol. 2001. PMID: 11329474 Free PMC article.
-
Cellular localization of Babesia bovis merozoite rhoptry-associated protein 1 and its erythrocyte-binding activity.Infect Immun. 2002 Oct;70(10):5822-6. doi: 10.1128/IAI.70.10.5822-5826.2002. Infect Immun. 2002. PMID: 12228313 Free PMC article.
-
High-level expression and purification of a truncated merozoite antigen-2 of Babesia equi in Escherichia coli and its potential for immunodiagnosis.J Clin Microbiol. 2003 Mar;41(3):1147-51. doi: 10.1128/JCM.41.3.1147-1151.2003. J Clin Microbiol. 2003. PMID: 12624044 Free PMC article.
-
Development of a stable transgenic Theileria equi parasite expressing an enhanced green fluorescent protein/blasticidin S deaminase.Sci Rep. 2021 Apr 27;11(1):9107. doi: 10.1038/s41598-021-88594-w. Sci Rep. 2021. PMID: 33907262 Free PMC article.
-
Roles of the Maltese cross form in the development of parasitemia and protection against Babesia microti infection in mice.Infect Immun. 2003 Jan;71(1):411-7. doi: 10.1128/IAI.71.1.411-417.2003. Infect Immun. 2003. PMID: 12496191 Free PMC article.
References
-
- Ali S, Sugimoto C, Kanemaru T, Kamada M, Onuma M. Characterization of epitope on an 18 kDa piroplasm surface protein of Babesia equi. J Protozool Res. 1995;5:47–57.
-
- Avarzed A, de Waal D T, Igarashi I, Saito A, Oyamada T, Toyoda Y, Suzuki N. Prevalence of equine piroplasmosis in Central Mongolia. Onderstepoort J Vet Res. 1997;64:141–145. - PubMed
-
- Avarzed A, Igarashi I, Kanemaru T, Hirumi K, Omata Y, Saito A, Oyamada T, Omata Y, Nagasawa H, Toyoda Y, Suzuki N. Improved in vitro cultivation of Babesia caballi. J Vet Med Sci. 1997;59:479–481. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brüning A. Equine piroplasmosis: an update on diagnosis, treatment and prevention. Br Vet J. 1996;152:139–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

