Contribution of subsaturating GABA concentrations to IPSCs in cultured hippocampal neurons
- PMID: 9651194
- PMCID: PMC6793480
- DOI: 10.1523/JNEUROSCI.18-14-05103.1998
Contribution of subsaturating GABA concentrations to IPSCs in cultured hippocampal neurons
Abstract
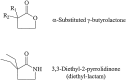
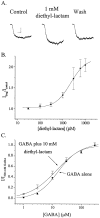
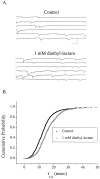
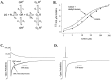
The time course of EPSCs and IPSCs is at least partly determined by the concentration profile of neurotransmitter acting on postsynaptic receptors. Several recent reports have suggested that the peak synaptic cleft concentration of the inhibitory neurotransmitter GABA likely reaches at least 500 microM, a level that saturates the GABAA receptor. In the course of investigating the experimental anticonvulsant 3,3-diethyl-2-pyrrolidinone (diethyl-lactam), we have observed an important contribution to IPSC decay by subsaturating concentrations of GABA. Diethyl-lactam augments currents elicited by the exogenous application of subsaturating concentrations of GABA in voltage-clamped, cultured hippocampal neurons and significantly prolongs the decay of autaptic IPSCs and miniature IPSCs in our cultures. In addition, diethyl-lactam potentiates currents in excised outside-out membrane patches elicited by the prolonged application of low concentrations of GABA. However, when patches are exposed to 1-2 msec pulses of 1 mM GABA, diethyl-lactam does not alter current decay. Tiagabine, which blocks GABA reuptake, does not prolong IPSCs, so it is unlikely that uptake inhibition accounts for the enhancement of IPSCs. EPSCs and miniature IPSC frequency are unaffected by diethyl-lactam, again consistent with a postsynaptic site of action. We propose that during an IPSC, a substantial number of postsynaptic receptors must be exposed to subsaturating concentrations of GABA. A simplified model of GABAA receptor kinetics can account for the effects of diethyl-lactam on exogenous GABA and IPSCs if diethyl-lactam has its main effect on the monoliganded states of the GABAA receptor.
Figures


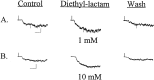
References
-
- Banks MI, Li T, Pearce RA. Effects of isoflurane on mIPSCs and excised neuronal GABAA receptors. Soc Neurosci Abstr. 1997;23:49.5.
-
- Braestrup C, Nielsen EB, Sonnewald U, Knutsen LJS, Andersen KE, Jansen JA, Frederiksen K, Andersen PH, Mortensen A, Suzdak PD. (R)-N-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]nipecotic acid binds with high affinity to the brain γ-aminobutyric acid uptake carrier. J Neurochem. 1990;54:639–647. - PubMed
-
- Canney DJ, Holland KD, Levine JA, McKeon AC, Ferrendelli JA, Covey DF. Synthesis and structure-activity studies of alkyl-substituted gamma-butyrolactones and gamma-thiobutyrolactones: ligands for the picrotoxin receptor. J Med Chem. 1991;34:1460–1467. - PubMed
-
- Choi DW, Farb DH, Fischbach GD. Chlordiazepoxide selectively potentiates GABA conductance of spinal cord and sensory neurons in cell culture. J Neurophysiol. 1981;45:621–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
