Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro
- PMID: 9651201
- PMCID: PMC6793488
- DOI: 10.1523/JNEUROSCI.18-14-05180.1998
Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro
Abstract
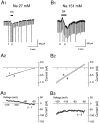
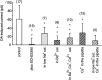
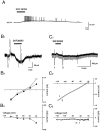
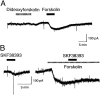
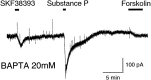
The aim of this study was to elucidate electrophysiologically the actions of dopamine and SKF38393, a D1-like dopamine receptor agonist, on the membrane excitability of striatal large aspiny neurons (cholinergic interneurons). Whole-cell and perforated patch-clamp recordings were made of striatal cholinergic neurons in rat brain slice preparations. Bath application of dopamine (1-100 microM) evoked a depolarization/inward current with an increase, a decrease, or no change in membrane conductance in a dose-dependent manner. This effect was antagonized by SCH23390, a D1-like dopamine receptor antagonist. The current-voltage relationships of the dopamine-induced current determined in 23 cells suggested two conductances. In 10 cells the current reversed at -94 mV, approximately equal to the K+ equilibrium potential (EK); in three cells the I-V curves remained parallel, whereas in 10 cells the current reversed at -42 mV, which suggested an involvement of a cation permeable channel. Change in external K+ concentration shifted the reversal potential as expected for Ek in low Na+ solution. The current observed in 2 mM Ba2+-containing solution reversed at -28 mV. These actions of dopamine were mimicked by application of SKF38393 (1-50 microM) or forskolin (10 microM), an adenylyl cyclase activator, and were blocked by SCH23390 (10 microM) or SQ22536 (300 microM), an inhibitor of adenylyl cyclase. These data indicate, first, that dopamine depolarizes the striatal large aspiny neurons by a D1-mediated suppression of resting K+ conductance and an opening of a nonselective cation channel and, second, that both mechanisms are mediated by an adenylyl cyclase-dependent pathway.
Figures




References
-
- Ajima A, Yamaguchi T, Kato T. Modulation of acetylcholine release by D-1, D-2 dopamine receptors in rat striatum under freely moving conditions. Brain Res. 1990;518:193–198. - PubMed
-
- Anderson JJ, Kuo S, Chase TN, Engber TM. Dopamine D1 receptor-stimulated release of acetylcholine in rat striatum is mediated indirectly by activation of striatal neurokinin-1 receptors. J Pharmacol Exp Ther. 1994;269:1144–1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
