Preferential utilization of acetate by astrocytes is attributable to transport
- PMID: 9651205
- PMCID: PMC6793490
- DOI: 10.1523/JNEUROSCI.18-14-05225.1998
Preferential utilization of acetate by astrocytes is attributable to transport
Abstract
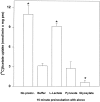
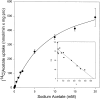
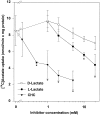
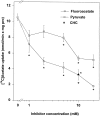
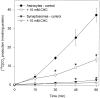
Exogenous acetate is preferentially metabolized by astrocytes in the CNS, but the biochemical basis for this selectivity is unknown. We observed that rat cortical astrocytes produce 14CO2 from 0.2 mM [14C]acetate at a rate of 0.43 nmol/min per milligram of protein, 18 times faster than cortical synaptosomes. Subsequent studies examined whether this was attributable to cellular differences in the transport or metabolism of acetate. The activity of acetyl-CoA synthetase, the first enzymatic step in acetate utilization, was greater in synaptosomes than in astrocytes (5.0 and 2.9 nmol/min per milligram of protein), indicating that slower metabolism in synaptosomes cannot be attributed to lack of enzymatic activity. [14C]Acetate uptake in astrocytes is rapid and time-dependent and follows saturation kinetics (Vmax, 498 nmol/min per milligram of protein; Km, 9.3 mM). Uptake is inhibited stereospecifically by L-lactate as well as by pyruvate, fluoroacetate, propionate, and alpha-cyano-4-hydroxycinnamate (CHC). Preloading astrocytes with L-lactate or acetate, but not D-lactate, pyruvate, or glyoxylate, transaccelerates [14C]acetate uptake. Acetate uptake by astrocytes appears to be mediated by a carrier with properties similar to that of monocarboxylate transport. In contrast, studies with synaptosomes provided no evidence for time-dependent, saturable, transaccelerated, or CHC-inhibitable uptake of [14C]acetate. The high rate of transport in astrocytes compared with synaptosomes explains the rapid incorporation of [14C]acetate into brain glutamine over glutamate. These findings provide support for the use of acetate as a marker for glial metabolism and suggest that extracellular acetate in the brain generated from acetylcholine and ethanol metabolism is accumulated first by astrocytes.
Figures



References
-
- Battaglioli G, Martin DL. Stimulation of synaptosomal γ-aminobutyric acid synthesis by glutamate and glutamine. J Neurochem. 1990;54:1179–1187. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin J-L, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT1) expressing Xenopus laevis oocytes. J Biol Chem. 1997;272:30096–30102. - PubMed
-
- Cerdan S, Künnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem. 1990;265:12916–12926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
