Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol
- PMID: 9651215
- PMCID: PMC6793471
- DOI: 10.1523/JNEUROSCI.18-14-05322.1998
Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol
Abstract
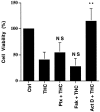
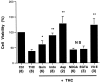
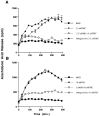
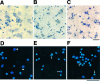
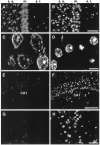
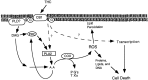
Marijuana consumption elicits diverse physiological and psychological effects in humans, including memory loss. Here we report that Delta9-tetrahydrocannabinol (THC), the major psychoactive component of marijuana, is toxic for hippocampal neurons. Treatment of cultured neurons or hippocampal slices with THC caused shrinkage of neuronal cell bodies and nuclei as well as genomic DNA strand breaks, hallmarks of neuronal apoptosis. Neuron death induced by THC was inhibited by nonsteroidal anti-inflammatory drugs, including indomethacin and aspirin, as well as vitamin E and other antioxidants. Furthermore, treatment of neurons with THC stimulated a significant increase in the release of arachidonic acid. We hypothesize that THC neurotoxicity is attributable to activation of the prostanoid synthesis pathway and generation of free radicals by cyclooxygenase. These data suggest that some of the memory deficits caused by cannabinoids may be caused by THC neurotoxicity.
Figures



References
-
- Abel EL. Marijuana and memory. Nature. 1970;227:1151–1152. - PubMed
-
- Abel EL. Retrieval of information after use of marijuana. Nature. 1971;231:58. - PubMed
-
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends Pharmacol Sci. 1992;13:201–206. - PubMed
-
- Audette CA, Burstein SH, Doyle SA, Hunter SA. G-protein mediation of cannabinoid-induced phospholipase activation. Pharmacol Biochem Behav. 1991;40:559–563. - PubMed
-
- Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
