Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord
- PMID: 9651218
- PMCID: PMC6793495
- DOI: 10.1523/JNEUROSCI.18-14-05354.1998
Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord
Abstract
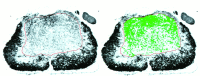
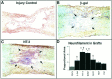
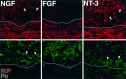
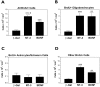
Functional loss after spinal cord injury (SCI) is caused, in part, by demyelination of axons surviving the trauma. Neurotrophins have been shown to induce oligodendrogliagenesis in vitro, but stimulation of oligodendrocyte proliferation and myelination by these factors in vivo has not been examined. We sought to determine whether neurotrophins can induce the formation of new oligodendrocytes and myelination of regenerating axons after SCI in adult rats. In this study, fibroblasts producing neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor, nerve growth factor, basic fibroblast growth factor, or beta-galactosidase (control grafts) were transplanted subacutely into the contused adult rat spinal cord. At 10 weeks after injury, all transplants contained axons. NT-3 and BDNF grafts, however, contained significantly more axons than control or other growth factor-producing grafts. In addition, significantly more myelin basic protein-positive profiles were detected in NT-3 and BDNF transplants, suggesting enhanced myelination of ingrowing axons within these neurotrophin-producing grafts. To determine whether augmented myelinogenesis was associated with increased proliferation of oligodendrocyte lineage cells, bromodeoxyuridine (BrdU) was used to label dividing cells. NT-3 and BDNF grafts contained significantly more BrdU-positive oligodendrocytes than controls. The association of these new oligodendrocytes with ingrowing myelinated axons suggests that NT-3- and BDNF-induced myelinogenesis resulted, at least in part, from expansion of oligodendrocyte lineage cells, most likely the endogenous oligodendrocyte progenitors. These findings may have significant implications for chronic demyelinating diseases or CNS injuries.
Figures





References
-
- Anderson T, Stokes BT. Experimental models for spinal cord injury research: physical and physiological considerations. J Neurotrauma. 1992;9[Suppl 1]:S135–S142. - PubMed
-
- Archelos JJ, Roggenbuck K, Schneider-Schaulies J, Linington C, Toyka KV, Hartung H-P. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J Neurosci Res. 1993;35:46–53. - PubMed
-
- Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. - PubMed
-
- Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978;39:254–266. - PubMed
-
- Bansal R, Pfeiffer SE. FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res. 1997;50:215–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
