Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation
- PMID: 9651231
- PMCID: PMC6793485
- DOI: 10.1523/JNEUROSCI.18-14-05508.1998
Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation
Abstract
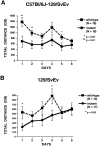
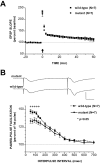
Spinocerebellar ataxia type 1 (SCA1) is a neurodegenerative disorder characterized by ataxia, progressive motor deterioration, and loss of cerebellar Purkinje cells. To investigate SCA1 pathogenesis and to gain insight into the function of the SCA1 gene product ataxin-1, a novel protein without homology to previously described proteins, we generated mice with a targeted deletion in the murine Sca1 gene. Mice lacking ataxin-1 are viable, fertile, and do not show any evidence of ataxia or neurodegeneration. However, Sca1 null mice demonstrate decreased exploratory behavior, pronounced deficits in the spatial version of the Morris water maze test, and impaired performance on the rotating rod apparatus. Furthermore, neurophysiological studies performed in area CA1 of the hippocampus reveal decreased paired-pulse facilitation in Sca1 null mice, whereas long-term and post-tetanic potentiations are normal. These findings demonstrate that SCA1 is not caused by loss of function of ataxin-1 and point to the possible role of ataxin-1 in learning and memory.
Figures



References
-
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. - PubMed
-
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. - PubMed
-
- Altemus KL, Almli CR. Neonatal hippocampal damage in rats: long-term spatial memory deficits and associations with magnitude of hippocampal damage. Hippocampus. 1997;7:403–415. - PubMed
-
- Banfi S, Servadio A, Chung M-y, Kwiatkowski TJ, Jr, McCall AE, Duvick LA, Shen Y, Roth EJ, Orr HT, Zoghbi HY. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet. 1994;7:513–519. - PubMed
-
- Banfi S, Servadio A, Chung M-y, Capozzoli F, Duvick LA, Elde R, Zoghbi HY, Orr HT. Cloning and developmental expression analysis of the murine homolog of the spinocerebellar ataxia type 1 gene (Sca1). Hum Mol Genet. 1996;5:33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
