B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice
- PMID: 9653093
- PMCID: PMC2525547
- DOI: 10.1084/jem.188.1.169
B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice
Abstract
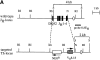
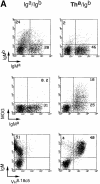
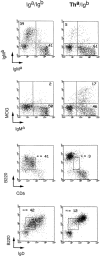
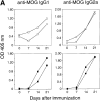
We studied the cellular basis of self tolerance of B cells specific for brain autoantigens using transgenic mice engineered to produce high titers of autoantibodies against the myelin oligodendrocyte glycoprotein (MOG), a surface component of central nervous system myelin. We generated "knock-in" mice by replacing the germline JH locus with the rearranged immunoglobulin (Ig) H chain variable (V) gene of a pathogenic MOG-specific monoclonal antibody. In the transgenic mice, conventional B cells reach normal numbers in bone marrow and periphery and express exclusively transgenic H chains, resulting in high titers of MOG-specific serum Igs. Additionally, about one third of transgenic B cells bind MOG, thus demonstrating the absence of active tolerization. Furthermore, peritoneal B-1 lymphocytes are strongly depleted. Upon immunization with MOG, the mature transgenic B cell population undergoes normal differentiation to plasma cells secreting MOG-specific IgG antibodies, during which both Ig isotype switching and somatic mutation occur. In naive transgenic mice, the presence of this substantial autoreactive B cell population is benign, and the mice fail to develop either spontaneous neurological disease or pathological evidence of demyelination. However, the presence of the transgene both accelerates and exacerbates experimental autoimmune encephalitis, irrespective of the identity of the initial autoimmune insult.
Figures









References
-
- Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. - PubMed
-
- Hardy RR, Carmack CE, Li YS, Hayakawa K. Distinctive developmental origins and specificities of murine CD5+B cells. Immunol Rev. 1994;137:91–118. - PubMed
-
- Nemazee DA, Buerki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-class I antibody genes. Nature. 1989;337:562–566. - PubMed
-
- Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. - PubMed
-
- Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, Weigert M. The site and stage of anti-DNA B cell deletion. Nature. 1995;373:252–255. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

