Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells
- PMID: 9653094
- PMCID: PMC2525549
- DOI: 10.1084/jem.188.1.181
Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells
Abstract
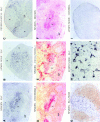
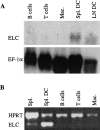
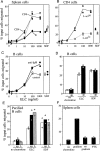
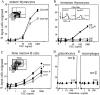
Movement of T and B lymphocytes through secondary lymphoid tissues is likely to involve multiple cues that help the cells navigate to appropriate compartments. Epstein-Barr virus- induced molecule 1 (EBI-1) ligand chemokine (ELC/MIP3beta) is expressed constitutively within lymphoid tissues and may act as such a guidance cue. Here, we have isolated mouse ELC and characterized its expression pattern and chemotactic properties. ELC is expressed constitutively in dendritic cells within the T cell zone of secondary lymphoid tissues. Recombinant ELC was strongly chemotactic for naive (L-selectinhi) CD4 T cells and for CD8 T cells and weakly attractive for resting B cells and memory (L-selectinlo) CD4 T cells. After activation through the B cell receptor, the chemotactic response of B cells was enhanced. Like its human counterpart, murine ELC stimulated cells transfected with EBI-1/CC chemokine receptor 7 (CCR7). Our findings suggest a central role for ELC in promoting encounters between recirculating T cells and dendritic cells and in the migration of activated B cells into the T zone of secondary lymphoid tissues.
Figures


References
-
- Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. - PubMed
-
- Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. - PubMed
-
- Niewenhuis P, Ford WL. Comparative migration of B- and T-lymphocytes in the rat spleen and lymph nodes. Cell Immunol. 1976;23:254–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

