Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I
- PMID: 9653099
- PMCID: PMC2525554
- DOI: 10.1084/jem.188.1.217
Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I
Abstract
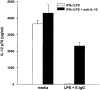
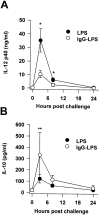
Macrophages can respond to a variety of infectious and/or inflammatory stimuli by secreting an array of proinflammatory cytokines, the overproduction of which can result in shock or even death. In this report, we demonstrate that ligation of macrophage Fcgamma receptors (FcgammaR) can lead to a reversal of macrophage proinflammatory responses by inducing an upregulation of interleukin (IL)-10, with a reciprocal inhibition of IL-12 production. IL-10 upregulation was specific to FcgammaR ligation, since the ligation of the Mac-1 receptor did not alter IL-10 production. The identification of the specific FcgammaR subtype responsible for IL-10 upregulation was determined in gene knockout mice. Macrophages from mice lacking the FcR gamma chain, which is required for assembly and signaling by FcgammaRI and FcgammaRIII, failed to upregulate IL-10 in response to immune complexes. However, mice lacking either the FcgammaRII or the FcgammaRIII were fully capable of upregulating IL-10 production, implicating FcgammaRI in this process. The biological consequences of FcgammaRI ligation were determined in both in vitro and in vivo models of inflammation and sepsis. In all of the models tested, the ligation of FcgammaR promoted the production of IL-10 and inhibited the secretion of IL-12. This reciprocal alteration in the pattern of macrophage cytokine production illustrates a potentially important role for FcgammaR-mediated clearance in suppressing macrophage proinflammatory responses.
Figures






References
-
- Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukocyte Biol. 1996;59:505–511. - PubMed
-
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

